Antecedentes: El objetivo del estudio fue evaluar la asociación entre el polimorfismo del gen C49620T ABCC8 y los parámetros antropométricos, bioquímicos, la función pancreática β-celular y la sensibilidad a la insulina entre los pacientes con enfermedad poliquística renal autosómica dominante (EPRAD). Métodos: Se incluyeron en el estudio 49 pacientes EPRAD (M/F: 19/30) y 50 controles sanos (M/F: 22/28) mayores de 18 años, con una función renal normal y sin diagnóstico de diabetes. Los genotipos ABCC8 (SUR1) C49620T (IVS15-3C/T, rs1799854) se determinaron utilizando una técnica de PCR-RFLP. Resultados: En el grupo de EPRAD entre los pacientes homocigotos TT, el contenido total de grasa corporal y el porcentaje de grasa en el peso corporal fue significativamente menor que entre los portadores del alelo C (16,1 ± 7,7 vs. 22,9 ± 7,1 kg, p = 0,04 y 22,8 ± 6,5 vs. 30,0 ± 6,1%, p = 0,001, respectivamente), mientras que el agua corporal total fue mayor (58,4 ± 4,3 vs. 53,7 ± 4,0 kg, p = 0,003). Entre los controles homocigotos TT se observaron mayores valores de índice de masa corporal y de colesterol LDL en comparación con los portadores de la variante C (26,3 ± 3,9 vs. 23,8 ± 3,4 kg/m2, p = 0,04 y 133,1 ± 27,0 vs. 114,3 ± 35,2 mg/dl, p = 0,05, respectivamente), así como mayor área bajo la curva de las concentración de glucosa (115,9 ± 23,9 vs. 102,7 ± 25,2 mmol * h/l, p = 0,046) durante una prueba de tolerancia a la glucosa oral. En el grupo de EPRAD y en los controles no se encontró asociación entre el polimorfismo investigado y la función secretora de las células pancreáticas β o sensibilidad a la insulina. Conclusión: El polimorfismo C49620T ABCC8 se asocia con factores de riesgo antropométricos para la diabetes tipo 2 entre los pacientes con EPRAD, con un efecto protector del genotipo TT, pero sin influencia en la función secretora de las células β del páncreas o en la sensibilidad a la insulina.
Background: The aim of the study was to evaluate an association between the C49620T ABCC8 gene polymorphism and anthropometric, biochemical parameters, pancreatic β-cell function and insulin sensitivity among autosomal dominant polycystic kidney disease (ADPKD) patients. Methods: Forty-nine ADPKD patients (M/F: 19/30) and fifty healthy controls (M/F: 22/28) aged above 18 years, with normal kidney function and no diagnosis of diabetes, were enrolled into the study. The ABCC8 (SUR1) C49620T (IVS15-3C/T, rs1799854) genotypes were determined using a PCR-RFLP technique. Results: In the ADPKD group among TT homozygous patients, total body fat content and percentage of fat in body weight were significantly lower than among C allele carriers (16.1±7.7 vs 22.9±7.1kg, p=0.04 and 22.8±6.5 vs 30.0±6.1%, p=0.001, respectively) while total body water was higher (58.4±4.3 vs 53.7±4.0kg, p=0.003). Among TT homozygous controls higher BMI values and LDL-cholesterol levels were observed if compared to C variant carriers (26.3±3.9 vs 23.8±3.4kg/m2 p=0.04 and 133.1±27.0 vs 114.3±35.2mg/dL, p=0.05, respectively), as well as higher area under curve of glucose concentrations (115.9±23.9 vs 102.7± 25.2mmol*h/L, p=0.046) during an oral glucose tolerance test. In the ADPKD group and among controls no association between the investigated polymorphism and secretory function of the pancreatic β cells or insulin sensitivity was found. Conclusion: The C49620T ABCC8 polymorphism is associated with anthropometric risk factors for type 2 diabetes among ADPKD patients, with a protective effect of the TT genotype, but without influence on pancreatic β-cell secretory function or insulin sensitivity.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease with a prevalence of 1:400 to 1:1000 live births among Caucasians.1 In most cases (85%) it is related to PKD1 gene mutations (chromosome 16), with the remainder caused by a mutation of the PKD2 gene (chromosome 4).2,3 In the Polish population the disease is associated with PKD1 mutations in 84% of the ADPKD affected families.4 PKD1 and PKD2 genes encode for polycystins 1 and 2.5 Biological properties of these cation channel proteins still remain under investigation.
Results of previously published research6-9 indicated that ADPKD is an independent risk factor of post-transplant diabetes mellitus (PTDM) development in the population of renal transplant recipients (RTRs). Our multicenter matched-pair study carried out in the population of the North-western Poland, investigating the incidence of PTDM in the ADPKD, RTRs population, did not confirm these results.10 In addition it must be noted that in a different study, on a large group (n=459) of ADPKD patients without kidney transplant it was found that type 2 diabetes mellitus is significantly less common when compared with their non-ADPKD siblings (1.7% vs 8.1%).11
Some researchers suggest that higher incidence of PTDM among ADPKD patients is associated with insulin resistance and compensatory hyperinsulinemia.12,13
The key role of the sulfonylurea receptor in the regulation of insulin secretion was shown in studies of patients with familial persistent hyperinsulinemic hypoglycemia of infancy, an autosomal recessive disorder characterized by unregulated hypersecretion of insulin due to ABCC8 (SUR1) gene mutations.14
It was also indicated that ABCC8 exon 16-3c/t polymorphism is associated with type 2 diabetes in Caucasians15,16 but independent research has not confirmed this finding.17,18
The aim of this work was to evaluate an association between the C49620T ABCC8 gene polymorphism (rs1799854) and anthropometric, biochemical parameters, as well as pancreatic β-cell function and insulin sensitivity among adult ADPKD patients with normal kidney function and no diagnosis of diabetes.
METHODS
The study group included 49 adult individuals with diagnosed ADPKD (19 males, 30 females) while the control group comprised 50 gender- and age-matched healthy individuals (22 males, 28 females). All subjects signed an informed consent before enrolment. Protocol of the study was approved by the Local Ethical Committee of the Pomeranian Medical University, Szczecin, Poland (approval number 001/135/06).
For the study group the following inclusion criteria were used: cysts present in both kidneys allowing a diagnosis of PKD phenotype according to Ravine,19 family history of ADPKD, serum creatinine concentration <1.35mg/dL and negative history of diabetes. As controls, individuals with negative family history of ADPKD, absence of cysts in the kidneys (Ravine’s criteria not fulfilled), serum creatinine concentration <1.35mg/dL and no prior diagnosis of diabetes, were enrolled.
At baseline full medical history was reviewed with clinical examination performed for every patient. Anthropometric parameters were recorded (body mass, height, waist and hip circumference). Based on these data both body mass index (BMI) and waist-to-hip ratio (WHR) were calculated. For BMI the weight/height2 formula was used.
Body fat and water content were measured using an infrared body composition analyser (Futrex 5000A/ZL, Futrex Inc., Hagerstown, USA).
An oral glucose tolerance test with 75g of glucose (OGTT) was performed on another day according to WHO guidelines.20 Before glucose administration (minute 0 of the test), venous blood was collected to measure fasting glucose, insulin, total cholesterol, LDL, HDL and triglyceride levels. Venous blood taken at the 30th, 60th, 90th and 120th minute of the test was assayed for concentrations of glucose and insulin. Analysis was performed by an enzymatic-amperometric method (Super GL; Dr Müller Gerätebau GmbH, Freital, Germany). Insulin concentration was measured using a microparticle enzyme immunoassay (AxSYM MEIA; Abbott Laboratories, Abbot Park, USA), while HbA1c was analysed by a immunoturbidometric method (Cobas Integra 800; Roche, Mannheim, Germany) for samples drawn on EDTA anticoagulant. For serum creatinine and lipid levels the Cobas Integra 800 bioanalyser was used.
The beta-cell function indexes were used in our study; ratios of insulin-to-glucose concentrations for each OGTT time point (INS/GLU 0, 30, 60, 90, 120), ratio of the area under curve of insulin (AUC Insulin) and glucose (AUC Glucose) concentrations (SECR AUC),21 homeostasis model assessment-% beta (HOMA%B),22,23 secretory 1st phase (SECR1P) and 2nd phase (SECR2P) calculated from the first 30 or 60 minutes of OGTT (SECR1P 30, SECR1P 60, SECR2P 30, SECR2P 60),21 index of beta cell function (INDXBETA),24 insulinogenic index (INSGENIN),25 and insulin sensitivity indexes: homeostasis model assessment-% sensitivity (HOMA%S),22,23 insulin sensitivity index (ISI) calculated from the fasting concentrations (ISI 0) or from 0 and 120 min of OGTT (ISI 120),21 insulin sensitivity composite index (ISI COMP),26 and Cederholm sensitivity index (ISI CEDE).27 In our recent study formulas of these indexes were presented.28
Genotyping
Genomic DNA was extracted from EDTA treated whole blood using QIAamp®DNA Mini Kit (Qiagen). The ABCC8 C49620T (rs1799854) genotype was determined using a PCR-RFLP technique, where 40ng of genomic DNA was amplified in a final volume of 20μl containing 10mM Tris-HCl (pH 8.3), 50mM KCl, 0.08% Nonidet P40, 200μM of each dNTP, 0.5U Taq polymerase and 4 picomoles of forward primer (5’-TTGGGTGCATCTGTCTGTCTGTCTTT-3’) and reverse primer (5’-AGCCCACCTGCCCCACGAT-3’). Amplification was performed as follows: an initial 5 minute denaturation at 94°C was followed by 32 cycles of PCR (denaturation at 94°C for 20s, annealing at 62°C for 40s, extension at 72°C for 40s) with final extension at 72°C for 8 minutes (GeneAmp® PCR System 9700, Applied Biosystems). The 122 bp amplicons of ABCC8 were digested by restriction enzyme – 10U PstI for 6h at 37°C. Restriction fragments were resolved on a 3% agarose gel stained with ethidium bromide and visualized in UV light (G:BOX Bioimaging System, Syngene). The C49620 allele was cleaved into 88 and 34bp restriction fragments, but T49620 allele remained uncleaved (122 bp). Genotypes were determined in two independent repeats.
Statistical analysis
As quantitative variables were not distributed normally, a Mann-Whitney test was used, while a Fisher exact test was implemented for qualitative variables. Results with p<0.05 were considered statistically significant. Data are presented as number (percentage) for qualitative variables or mean±standard deviation for quantitative variables. Statistica 7.1 software was used for all statistical analyses.
RESULTS
In the ADPKD group the distribution of studied genotypes was as follows: CC genotype found in 39%, CT in 43% and TT in 18% of patients. In the control group CC genotype was found in 18%, CT in 56% and TT in 26% subjects. The distributions differed with borderline significance (χ2=5.29, p=0.071), but the proportion of T carriers (CT+TT genotypes) was significantly lower in the ADPKD group (61% vs 82%, p=0.027), and the T allele was significantly less frequent in ADPKD patients than in controls (40% vs 54%, p=0.048). Both distributions were consistent with Hardy-Weinberg equilibrium (p>0.5).
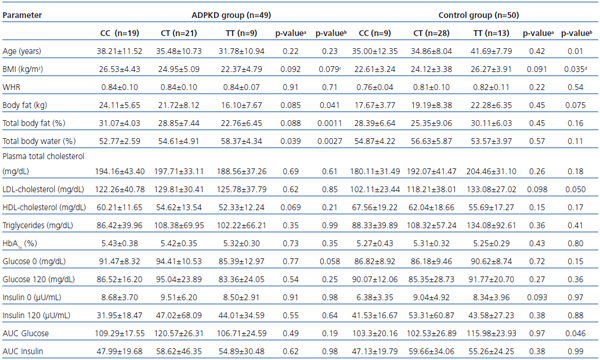
Association between anthropometric and biochemical parameters of the patients with ADPKD, controls and C49620T genotype are presented in table 1.
Among ADPKD patients total body fat content and percentage of fat in body weight were significantly lower in TT homozygotes, with BMI lower as well (borderline significance), while total body water content was significantly higher than in C allele carriers (CC+CT).
BMI difference between TT and CC homozygotes proved to be of statistical significance (p=0.042). Among T allele carriers (TT+CT) tendency for lower body fat content, percentage of fat in body weight and BMI if compared to CC homozygous individuals was on the borderline of statistical significance (0.1
Among ADPKD patients no significant association between biochemical parameters and genotype was found. However fasting glucose levels among TT homozygous subjects were lower (borderline significance) if compared to C allele carriers, and HDL-cholesterol levels were lower (borderline significance) among T allele carriers if compared to CC homozygotes.
In the control group TT homozygous subjects were significantly older than C allele carriers (CC+CT) with higher BMI and total body fat mass (borderline statistical significance). Similarly, for the T allele (TT+CT), only slightly higher BMI values were observed if compared to CC homozygotes (borderline statistical significance). For all the biochemical parameters analysed in the control group only values of the area under curve for glucose (AUC glucose) during an OGTT, and LDL-cholesterol concentration among TT homozygotes if compared to the C allele carriers (CC+CT) were significantly different. LDL-cholesterol and fasting insulin levels were higher (borderline significance) in T allele carriers than in CC homozygotes.
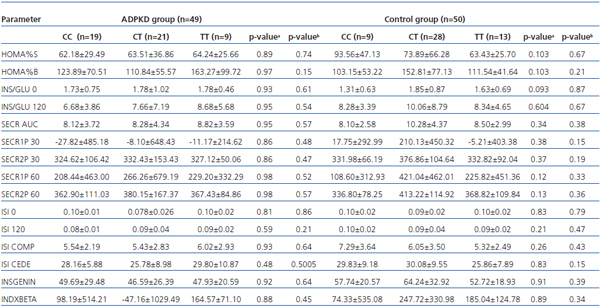
Association between the C49620T variant and both indices of pancreatic β-cell secretory function and insulin sensitivity, among ADPKD patients and controls are presented in table 2.
In the analysed groups no significant association between the investigated indices and genotype was found. In the control group only the fasting insulin/glucose ratio was higher among T allele carriers than among CC homozygotes with borderline significance.
DISCUSSION
Insulin resistance and obesity are the underlying causes of type 2 diabetes and therefore much interest is focused on the potential genes involved.29 The results of novel research have proven that ADPKD among patients with normal kidney function is associated not only with hypertension but also with higher prevalence of abdominal obesity and higher fasting glycemia.30 Nevertheless, diabetes mellitus type 2 was found more rarely in these subjects.11 Our preliminary study is the first association analysis of the ABCC8 intronic C49620T transition and anthropometry as well as lipid and glucose metabolism parameters in a homogenous group of ADPKD patients.
C49620T ABCC8 genotype distribution in our ADPKD group (39% CC, 43% CT and 18% TT) was similar to the one reported by Gloyn et al.,17 in a large UK cohort (31% CC, 52% CT, 17% TT in the non insulin dependent diabetes mellitus group, (n=1182, and 34% CC, 48% CT, 18% TT in the control group, n=854), where no association of this polymorphism with diabetes mellitus was detected (p=0.63). However, in our control group the T allele was more frequent (18% CC, 56% CT and 26% TT), similarly to that in the nondiabetic Caucasian subjects studied by Inoue et al.15 (21% CC, 50% CT, 29% TT).
The most significant statistical associations in our study were identified for the body fat and water content. In the ADPKD group the body fat percentage was lower and that of body water higher among TT homozygous patients. This feature was the most pronounced among carriers of two T alleles because the comparison of joint T allele carriers (TT+CT) with the CC homozygotes significantly reduced significance of the associations observed. Conversely to the ADPKD group, in the control group higher body fat content was observed among TT homozygous individuals when compared to C allele carriers, with the result of borderline significance. No association between the C49620T ABCC8 SNP and body water/fat content has been described so far.
Additionally, in this study it was found that in the ADPKD group mean BMI was 4.16kg/m2 lower among TT homozygous individuals than CC homozygotes. However, in the control group the BMI values among TT homozygotes was 3.66kg/m2 higher if compared to the CC homozygotes. No association between any genotype and WHR or waist circumference was found (data not shown). Similar results were obtained in a study by Stefański et al.,31 with BMI significantly lower (by 0.7kg/m2) among TT homozygous patients with obesity and long-lasting type 2 diabetes if compared to CC homozygotes. On the other hand in the studies by Hansen et al.32, Hart et al.33 and Meirhaeghe et al.34 no statistically significant differences in BMI and WHR between carriers of the different exon 16 -3C/T genotypes were found.
HDL-cholesterol levels among T allele carriers (TT+TC) were lower with borderline significance than in CC homozygotes only in the ADPKD group. In the control group no differences in HDL-cholesterol levels were found between genotypes, but the LDL-cholesterol concentration was significantly higher among TT homozygotes than among C allele carriers. However Stefański et al.31 found the HDL-cholesterol to be higher among CT heterozygous patients than among TT or CC homozygotes. This discordance might be related to the fact that most of the patients in the study performed by Stefański et al. were treated with fibrates or statins due to dyslipidaemia; in our study none of the patients used such medications. Meirhaeghe et al.34, in a study of patients with type 2 diabetes treated with sulfonylureas, has shown that fasting plasma triglyceride concentrations were higher by 35% in TT homozygotes than in C-allele carriers. These authors did not investigate HDL and LDL-cholesterol levels but, as in the results of our study, total cholesterol serum concentration proved not to be associated with any of the genotypes.
Analysis of carbohydrate metabolism parameters has revealed that in the ADPKD group TT homozygotes had lower fasting glucose levels (of borderline statistical significance) while TT controls had notably higher AUC glucose values if compared to the C allele carriers. In the study by Hart et al.33 no differences were found between exon 16 genotypes as regards fasting glucose or insulin levels in subjects with impaired glucose tolerance. In our ADPKD and control groups, no association of the C49620T variant with fasting insulin concentration was found.
Similarly to Hart et al. [33], no association between exon 16 genotypes and HbA1c levels was found. Nikolac et al.,35 have found that in Caucasian patients with type 2 diabetes the TT genotype was associated with significant elevation of HbA1c concentration in patients on sulfonylurea therapy, but there was no difference in fasting and postprandial glucose concentrations. Patients enrolled for our study did not receive hypoglycemic medications.
No association between C49620T polymorphism and secretory function of the pancreatic β-cell during OGTT was found. This result supports the similar observation of Stefański et al.,30 who analysed parameters of an intravenous glucagon stimulatory test (glucose, C-peptide, HOMA2-%B, HOMA2-%S, HOMA2IR). Hansen et al.32 reported no association between the C49620T genotype and glucose-induced or tolbutamide-induced serum insulin and C-peptide responses among healthy subjects. Also Weisnagel et al.,36 in a study of non-diabetic French Caucasians, indicated a lack of association between C49620T genotype and fasting plasma insulin, OGTT glucose and insulin response.
In contrast to the results obtained in this study and other researchers, Hart et al.33 showed that in subjects with both normal and impaired glucose tolerance (IGT) from the Netherlands, the 49620T allele is associated with impaired second-phase insulin secretion during a hyperglycemic clamp. In the IGT group, a significant increase in the proinsulin to insulin ratio was observed in carriers of the T allele compared with the CC genotype. First phase insulin secretion, insulin sensitivity (ISI, as measured with the glucose clamp), and fasting insulin were similar between T carriers and non-carriers. Elbein et al.,37 when investigating nondiabetic members of familial type 2 diabetic kindred who had undergone tolbutamide-modified intravenous glucose tolerance test, indicated that the C49620T genotype was a significant determinant of both DI (a measure of beta-cell compensation for insulin sensitivity) and an analogous index based on acute insulin response to tolbutamide. Heterozygous individuals (CT) showed the lowest indices, suggesting beta-cell function impairment whereas the DI in the two homozygous states (CC and TT) did not differ significantly. Their data support an association of ABCC8 C49620T variants with beta-cell function, measured either as acute insulin response to glucose or tolbutamide, but no specific direction of the allelic influence is described.
This study indicates that C49620T variants do not exert an influence on β-cell secretory function as measured by an OGTT and is concordant with results obtained by Weisnagel et al. (OGTT),36 Stefański et al. (iv glucagon stimulatory test)31 and Hansen et al. (tolbutamide-modified intravenous glucose tolerance test).32 However, it must be noted that use of the hyperglycemic clamp by Hart et al. (2000) revealed that C49620T alleles are associated with the reduced second phase insulin secretion.
In patients with ADPKD, hypothetical influence of the mutated protein (polycystin 1 or 2) on the function of ATP-sensitive potassium (KATP) channels of pancreatic β-cells could modify its response to glucose. In our recent study28 it was shown that among ADPKD patients an impairment of the first phase insulin secretion during OGTT is observed. It seems possible that interaction between polycystin gene mutations and ABCC8 polymorphism is present, which results in the favorable effect of the 49620T allele on the lipid and glucose metabolism and morphometric parameters in ADPKD patients but not in the individuals with fully functional polycystins. T allele frequency among ADPKD patients was lower than among controls, which might be a reason for higher incidence of metabolic syndrome observed in this group.30 However to confirm the hypothesis on the interaction of the polycystin gene mutations and ABCC8 polymorphism further studies on larger groups with inclusion of other functional ABCC8 variants are necessary.
CONCLUSIONS
The results obtained in this preliminary study reveal the association between C49620T ABCC8 polymorphism and anthropometric risk factors for type 2 diabetes (higher BMI and higher content of the body fat) among patients with ADPKD, with a protective effect of the TT genotype. However, neither the TT genotype nor T allele exerted such a protective effect in the control group. In the analysed groups no association between the C49620T ABCC8 polymorphism and β-cell secretory function or insulin resistance parameters measured during OGTT was found.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Table 1. Associations between C49620T (rs1799854) variants of the ABCC8 gene and anthropometric and biochemical parameters among ADPKD and control groups
Table 2. Associations between C49620T (rs1799854) variants of the ABCC8 gene and insulin resistance and beta-cell secretory function parameters among ADPKD and control groups