Microalbuminuria is a common clinical symptom that manifests in the early stages of diabetic kidney disease (DKD) and is also the main feature of glomerular endothelial cells (GECs) injury. There is increasing evidence that the transcytosis of albumin across GECs is closely related to the formation of albuminuria. Our previous studies have shown that angiopoietin 2 (ANGPT2) can inhibit albumin transcytosis across renal tubular epithelial cells by activating caveolin 1 (CAV1) phosphorylation during high glucose (HG) exposure. The role of ANGPT2 in albumin transcytosis across GECs remains unclear. Losartan significantly reduces albuminuria, but the mechanism has not been clarified.
MethodsWe established an in vitro albumin transcytosis model to investigate the change in albumin transcytosis across human renal glomerular endothelial cells (hrGECs) under normal glucose (NG), high glucose (HG) and losartan intervention. We knocked down ANGPT2 and CAV1 to evaluate their roles in albumin transcytosis across hrGECs and verified the relationship between them. In vivo, DKD mouse models were established and treated with different doses of losartan. Immunohistochemistry and Western blot were used to detect the expression of ANGPT2 and CAV1.
ResultsIn vitro, the transcytosis of albumin across hrGECs was significantly increased under high glucose stimulation, and losartan inhibited this process. The expression of ANGPT2 and CAV1 were both increased in hrGECs under HG conditions and losartan intervention reduced the expression of them. Moreover, ANGPT2 downregulation reduced albumin transcytosis in hrGECs by regulating CAV1 expression. In vivo, the expression of ANGPT2 and CAV1 in the glomerulus was both increased significantly in DKD mice. Compared with DKD mice, losartan treatment reduced albuminuria and decreased the expression of ANGPT2 and CAV1 in a dose-dependent manner.
ConclusionsANGPT2 exacerbated albumin transcytosis across GECs by increasing CAV1 expression during HG exposure, thereby increasing albuminuria. Losartan reduces albumin transcytosis and albuminuria formation in DKD by inhibiting the upregulation of ANGPT2 under HG conditions. Our findings suggest that ANGPT2 and CAV1 may be novel therapeutic targets for diabetic albuminuria. In addition, we provide new evidence to elaborate on the mechanism of losartan in the development of DKD.
La microalbuminuria es un síntoma clínico común que se manifiesta en las fases tempranas de la enfermedad renal diabética (ERD), y también es característica del daño de las células endoteliales glomerulares (GEC). Existe evidencia creciente en cuanto a que la transcitosis de la albúmina a través de las GEC está estrechamente relacionada con la formación de albuminuria. Nuestros estudios previos reflejaron que angiopoyetina 2 (ANGPT2) puede inhibir la transcitosis de la albúmina a través de las células epiteliales tubulares renales activando la fosforilación de caveolina 1 (CAV1) durante la exposición a hiperglucemia (HG). El rol de ANGPT2 en la transcitosis de la albúmina a través de las GEC resulta incierto. Losartan reduce considerablemente la albuminuria, aunque no se ha esclarecido el mecanismo.
MétodosEstablecimos un modelo in vitro de transcitosis de la albúmina para investigar el cambio de dicho mecanismo a través de las células endoteliales glomerulares renales humanas (hrGEC) en condiciones de glucosa normal (GN), hiperglucemia (HG) e intervención de losartan. Realizamos breakdown de ANGPT2 y CAV1 para evaluar sus roles en la transcitosis de la albúmina a través de las hrGEC, y verificamos la relación entre ellas. Se establecieron modelos in vivo de ratones con ERD, tratados con diferentes dosis de losartan. Se utilizaron pruebas de inmunohistoquímica e inmunotransferencia para detectar la expresión de ANGPT2 y CAV1.
ResultadosIn vitro, la transcitosis de la albúmina a través de hrGEC se incrementó considerablemente en condiciones de estimulación de la hiperglucemia, inhibiendo losartan este proceso. La expresión de ANGPT2 y CAV1 se incrementó en las hrGEC en condiciones de HG, reduciendo la intervención de losartan la expresión de ambas. Además, la regulación decreciente de ANGPT2 redujo la transcitosis de la albúmina en las hrGEC, regulando la expresión de CAV1. In vivo, la expresión de ANGPT2 y CAV1 en los glomérulos se incrementó considerablemente en los ratones con ERD. En comparación con los ratones con ERD, el tratamiento de losartan redujo la albuminuria y disminuyó la expresión de ANGPT2 y CAV1 dependiendo de la dosis.
ConclusionesANGPT2 exacerbó la transcitosis de la albúmina a través de las GEC, incrementando la expresión de CAV1 durante la exposición a HG, y aumentando por tanto la albuminuria. Losartan reduce la transcitosis de la albúmina y la formación de albuminuria en la ERD, inhibiendo la regulación creciente de ANGPT2 en condiciones de HG. Nuestros hallazgos sugieren que ANGPT2 y CAV1 pueden ser objetivos terapéuticos noveles para la albuminuria diabética. Además, aportamos evidencia nueva para elaborar el mecanismo de losartan en el desarrollo de la ERD.
Diabetic kidney disease (DKD) is one of the most common serious microvascular complications of diabetes mellitus. Globally, DKD has become the leading cause of end-stage renal disease (ESRD).1,2 Compared with non-DKD ESDR patients, DKD ESRD patients have a higher mortality rate.3 Microalbuminuria is a common clinical symptom in the early stages of DKD and is also the main feature of glomerular endothelial cells (GECs) injury.4,5
Under physiological conditions, albumin transcytosis, an important method of albumin transfer from the bloodstream to the interstitium, plays an essential role in maintaining cell and body homeostasis.6 In the lung, stimulation of albumin transcytosis by proinflammatory mediators may be involved in the process of alveolar protein leakage during lung injury.7 Another study showed that receptor-mediated albumin transcytosis exists in dermal microvascular endothelial cells.8 The molecular mechanisms and signaling pathways of albumin transcytosis are different in different tissues.
The glomerular filtration barrier (GFB) is formed from three layers: glomerular endothelial cells (GECs), the glomerular basement membrane (GBM), and podocytes.9 Traditionally, albuminuria is mainly caused by loss of GFB integrity and proximal tubule reabsorption defects. In recent years, however, there has been increasing evidence that the transcytosis of albumin across GECs, podocytes, and renal tubular epithelial cells is closely related to the formation of albuminuria during the early phases of DKD.10–12 Exploring the mechanisms of albumin transcytosis may highlight therapeutic targets for preventing or delaying pathological albuminuria.
Previous studies have shown that caveolae, as a metabolic platform that mediates the endocytosis of albumin, cholesterol, and glucose, play an important role in albumin endocytosis and subsequent transcytosis.13–16 CAV1 is one of the major scaffold proteins involved in the formation of caveolae.17,18 CAV1 deficiency in mice resulted in the abnormal uptake and transport of albumin in endothelial cells.19,20 The C-terminal domain of CAV1 contains ubiquitination sites, whereas its N-terminal domain contains phosphorylation sites.21 Increased CAV1 phosphorylation significantly increases albumin transcytosis in GECs.11
Angiopoietin 2 (ANGPT2), a member of the endothelial growth factor family, plays an important role in kidney disease.22 ANGPT2 in plasma and urine is increased with renal damage in patients with type 2 diabetes mellitus and is associated with albuminuria.23,24 In normal mature glomeruli, the expression of ANGPT2 is low or undetectable but is upregulated in hyperglycemia and immune-mediated glomerulopathy.25–28 The expression of ANGPT2 in the kidney of a DKD animal model is significantly increased.29 This context indicates that ANGPT2 plays an important role in the progression of DKD albuminuria.
Our previous study showed that the upregulation of ANGPT2 expression can inhibit albumin transcytosis across renal tubular epithelial cells by activating CAV1 phosphorylation during HG exposure, thereby increasing albumin excretion.12 The role of ANGPT2 and the relationship between ANGPT2 and CAV1 in albumin transcytosis across GECs has not yet been studied. Losartan (Los), as the first clinically used selective angiotensin subtype 1 receptor antagonist, can significantly reduce renal angiotensin II content and albuminuria.30,31 However, no studies have examined the role of losartan in albumin transcytosis in the development of DKD.
The aim of this study was to determine the roles of ANGPT2 and CAV1 in albumin transcytosis across GECs under HG conditions. In addition, we explored the mechanism by which losartan reduces albuminuria in DKD.
Materials and methodsMouse modelsMale C57BL/6J mice (8 weeks old; 20–25g) were provided by HFK (Bioscience Co., Ltd., Beijing, China). All mice were maintained under standard feeding conditions (temperature 23°C; 12h light and 12h dark cycle) and had ad libitum access to food and water. Our research was performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and the Animal Welfare Act in China. All protocols were approved by the Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science and Technology (Permit Number: 2019S2570).
All mice were randomly assigned to the control group (Ctrl, n=6) or the diabetic group (n=18). The diabetic group was induced by a single intraperitoneal injection of 150mg/kg streptozotocin (STZ, Boster Biological Technology, Wuhan) (dissolved in citrate buffer), as previously described.12,32 The control group received an equal volume of citrate buffer. Diabetes was diagnosed if the tail blood glucose concentration of the mice was ≥16.7mmol/L for 3 consecutive days. 4 to 6 weeks after STZ treatment, mouse urine was collected to measure the volume and protein concentration. DKD was verified by urine protein≥30mg/24h. Subsequently, all DKD mice were randomly divided into the DKD group (n=6), DKD+ low-dose losartan treated group (DKD+Los-L, n=6), and DKD+ high-dose losartan treated group (DKD+Los-H, n=6). The DKD+ Los-L group and DKD+ Los-H group were intragastrically administered 10mg/kg/d and 50mg/kg/d losartan, respectively. After 12 weeks treatment,33 urine samples were collected to measure 24-h urinary protein and urinary creatinine (Ucr), and blood samples were collected to measure serum creatinine (Scr) and blood urea nitrogen. Mice were euthanized for tissue harvest.
Cell culturehrGECs were purchased from Beijing Beina Chuanglian Biotechnology Research Institute. hrGECs were cultured in Delbuco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and 100U/mL penicillin–streptomycin at 37°C and 5% CO2 in a humidified atmosphere. Cells were divided into different groups as follows: normal glucose group (NG): 5.5mmol/L d-glucose; high glucose group (HG): 40mmol/L d-glucose; HG+1μmol/L losartan group (HG+Los-L); HG+10μmol/L losartan group(HG+Los-H).
Albumin uptake analysisFITC-labeled albumin uptake was analyzed as previously described.11,12 hrGECs were incubated with 50μg/ml or 100μg/ml FITC-BSA for 1h or 3h after treatment with different agents in NG or HG conditions. The cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100/PBS for 20min, and stained with DAPI to reveal the nuclei. After covering with a coverslip, images were taken with a fluorescence microscope (Olympus BX51, Tokyo, Japan). ImageJ was used to analyze the integrated fluorescence intensities.
Monolayer integrity for the transcytosis assayhrGECs were planted in a 24-well Transwell plate at a suitable density and cultured in a constant temperature incubator at 37°C. Upon reaching 100% confluence, the transendothelial electrical resistance (TEER) value of each well was measured by EVOM2™ (WPI, New Haven, CT, USA) as previously described.12 The measurement was repeated 9–12 times in each well, and the average value was taken. The medium group without cells inoculated with the same glucose concentration was used as the blank group. The integrity of the cell monolayer was proven when TEER reached a plateau. The TEER value of hrGECs reached a plateau after 96h of culture, when the TEER value reached approximately 40Ωcm2.
Albumin transcytosis assayThe determined hrGECs were resuspended in 5.5mmol/L or 40mmol/L d-glucose, then moved to another Transwell 24-well plate and incubated for 12h. Then, hrGECs were incubated with 50μg/ml or 100μg/ml FITC-BSA in the upper chambers of the Transwell, while the medium was mixed with BSA in the lower chambers of the Transwell. The fluorescence signals in the upper and lower chambers of the Transwell were measured by a fluorescence spectrophotometer (Infinite F200PRO; T ecan, Männedorf, Switzerland).
Histological analysis and immunohistochemistry stainingKidney tissue was cut lengthwise along the long axis, fixed with paraformaldehyde, embedded in paraffin, and cut into 4μm thick slices several times. To evaluate the degree of pathological damage to kidney tissues, the sections were stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS).
For immunohistochemistry staining, kidney sections were incubated in EDTA at 120°C for 5min and blocked with 3% H2O2 for 15min. Then, nonspecific sites were blocked with 5% normal goat serum for 30min at room temperature. The slices were incubated overnight at 4°C with anti-angiopoietin 2 (ab153934; Abcam, Cambridge, MA, USA) and anti-caveolin-1 (CST3238; CST, Danvers, MA, USA) antibodies. Then, the sections were incubated with a biotinylated goat anti-rabbit lgG secondary antibody (Beyotime, Jiangsu, China) for 20min. After counterstaining with 3,3′-diamnobenzidine (DAB, EnVision Detection Kit), slices were dehydrated via an alcohol gradient, and images were observed by a light microscope.
RNA transfectionTo knockdown CAV1, small interfering RNA (siRNA)-CAV1 (si-CAV1) and a normal control siRNA (si-NC) were designed and synthesized by RiboBio (Guangzhou, China). When hrGECs reached 30–40% confluence, the medium was replaced with Opti-MEM (Gibco, Thermo Fisher Scientific, MA, USA). Subsequently, cells were transfected with these siRNAs using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) diluted in OPTI-MEM. After 4–6h, OPTI-MEM was replaced with normal medium and incubated for 48h with 5.5mmol/L or 40mmol/L d-glucose. Then, the cells were harvested for further experiments.
To knockdown ANGPT2, lentiviruses expressing shRNA targeting ANGPT2 (shANGPT2) and the corresponding control vector (scra) were designed and synthesized by Genechem (Shanghai, China). Cells were transfected according to the manufacturer's instructions. After 48h of transfection, fluorescence microscopy (Nikon, Tokyo, Japan) was used to observe the transduction efficiency.
Western blot analysisKidney tissue and hrGECs were collected and lysed in ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Jiangsu, China). The protein concentration was measured using a BCA assay kit. Approximately 50μg protein samples were loaded in 8–12% SDS–PAGE gels and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). After blocking with 5% nonfat dried skimmed milk (Beyotime) for 1h at room temperature, the membranes were incubated overnight at 4°C with antibodies against GAPDH (1:5000, ab8245, Abcam), angiopoietin 2 (1:1000, ab153934; Abcam) and caveolin-1 (1:1000, CST3238; CST). Finally, the membranes were incubated with corresponding secondary antibodies (1:2500; Eric Biotechnology, Wuhan, China) for 1h at room temperature before detection.
Statistical analysesAll experiments were performed more than three times. The results are expressed as the mean±standard error of the mean (SEM) by GraphPad Prism 7.0 software. Student's t test was used to analyze statistical significance between the two conditions. One-way analysis of variance (ANOVA) was used for multiple group comparisons followed by Bonferroni's post hoc test. Significance was set at P<0.05.
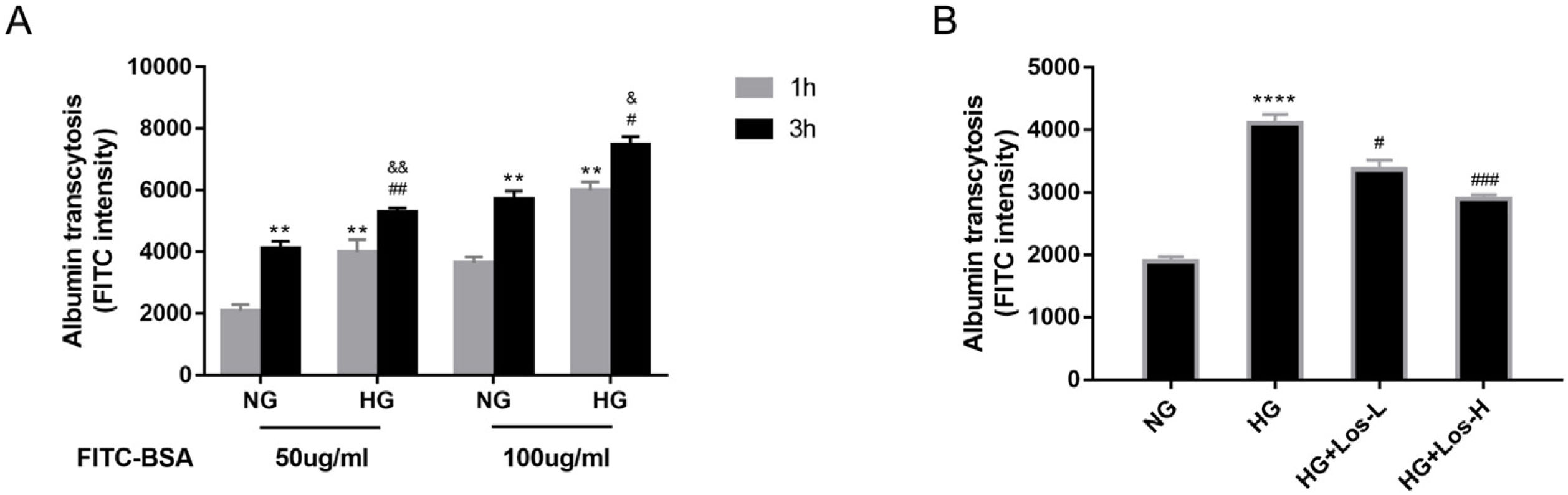
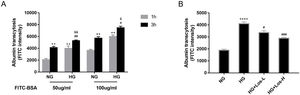
ResultsHigh glucose increased albumin transcytosis across hrGECs and losartan inhibited this processIn vitro, we used the albumin transcytosis model to measure the transport of 50μg/ml and 100μg/ml FITC-BSA across the monolayer of hrGECs cultured under normal glucose and high glucose conditions, respectively. As Fig. 1A shows, albumin transcytosis increased with incubation time and the concentration levels of FITC-BSA. The transcytosis of albumin was significantly increased under high glucose stimulation in hrGECs. Losartan administration reduced the transcytosis of albumin across hrGECs under HG conditions in a concentration-dependent manner (Fig. 1B).
High glucose increased albumin transcytosis across hrGECs and losartan inhibited this process. (A) Concentration-dependent transcytosis of FITC-albumin at 1 and 3h in NG (5.5mmol/L d-glucose) condition and HG (40mmol/L d-glucose) condition. **P<0.01 vs. 1h NG 50μg/ml group, #P<0.05 vs. 3h NG 50μg/ml or NG 100μg/ml, &P<0.05 vs. 1h HG 50μg/ml or HG 100μg/ml group. (B) Transcytosis of FITC-albumin in hrGECs incubated with NG, HG, HG+Los-L(1μmol/L), and HG+Los-H(10μmol/L). ****P<0.0001 vs. NG group, #P<0.05 or ###P<0.001 vs. HG group. Data are presented as means±SEM.
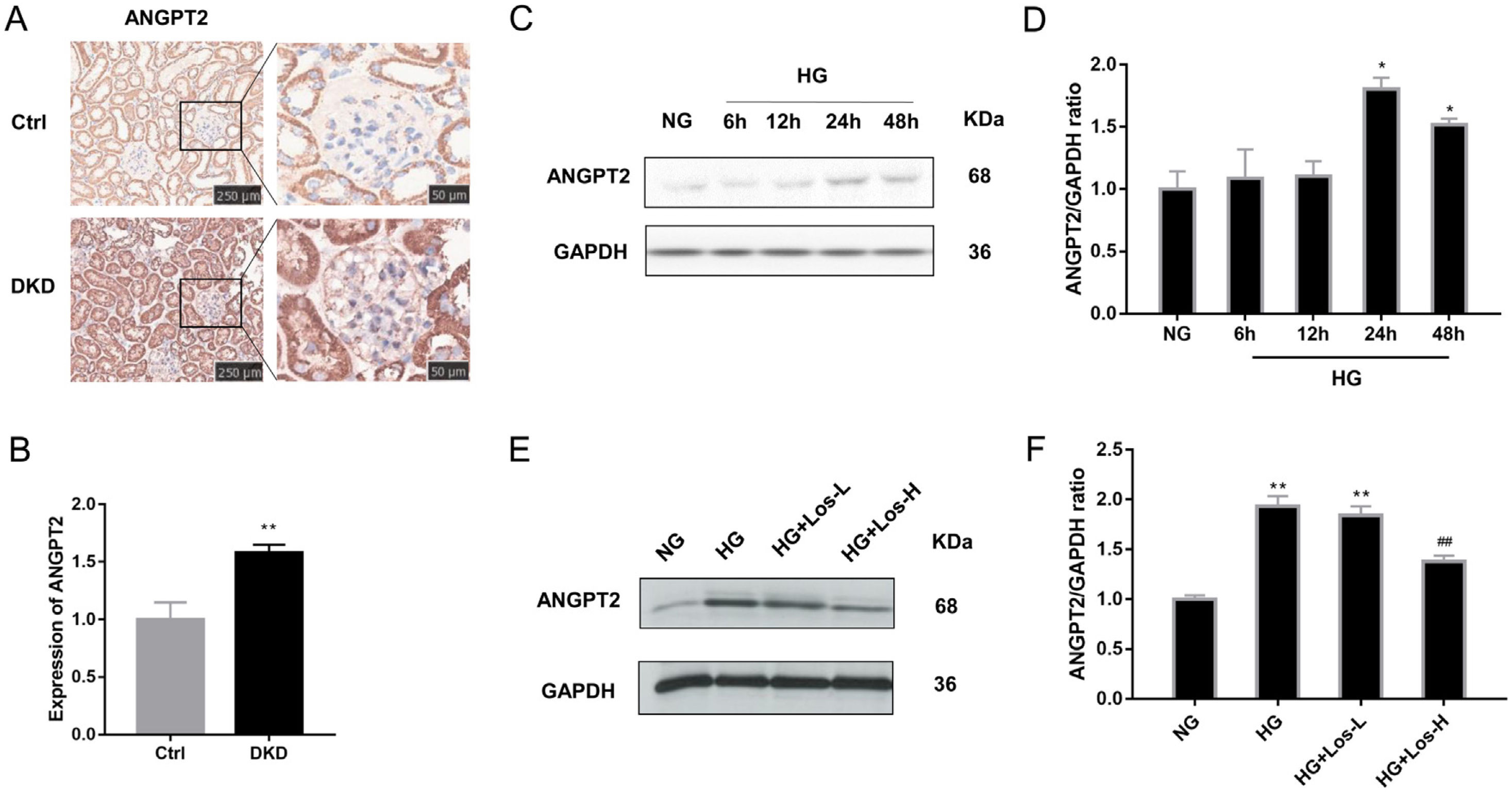
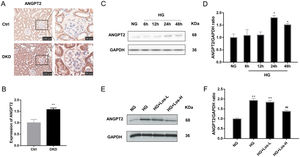
Immunohistochemical staining results showed that the expression of ANGPT2 in the glomerulus was increased significantly in DKD mice (Fig. 2A, B). In vitro, the expression of ANGPT2 was also increased in hrGECs under HG conditions (Fig. 2C, D). Losartan treatment significantly reduced the expression of ANGPT2 in hrGECs under HG stimulation (Fig. 2E, F). These results indicate that ANGPT2 is involved in the pathological process of GECs under high glucose stimulation, and losartan could regulate this process.
HG up-regulated ANGPT2 expression and losartan inhibited this process. (A) Representative immunohistochemical (IHC) staining of ANGPT2 in kidney. (B) Statistical data of IHC staining of ANGPT2 (n≥5). **P<0.01 vs. Control. (C) Representative western blot of ANGPT2; (D) Densitometric analysis of ANGPT2 western blot signals (n≥3). *P<0.05 vs. NG. (E) Western blot of ANGPT2 of hrGECs incubated with NG, HG, HG+Los-L, HG+ Los-H. (F) Densitometric analysis of western blot signals of ANGPT2 (n≥3). **P<0.01 vs. NG group, ##P<0.01 vs. HG group. Data are presented as means±SEM.
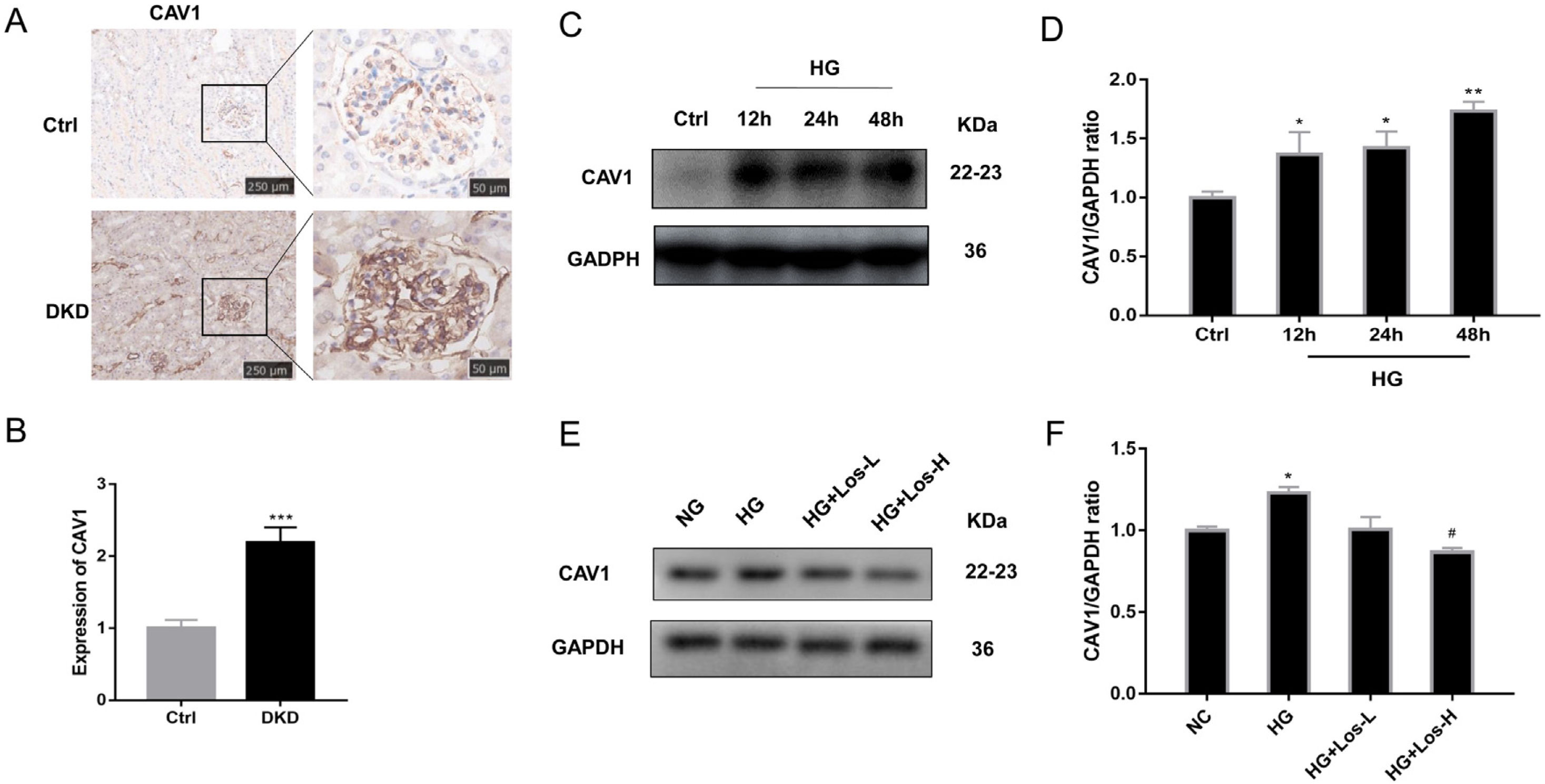
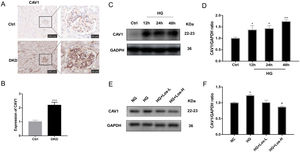
Previous studies have found that CAV1 is closely related to the production of albuminuria in DKD.12,34 Compared with control mice, the expression of CAV1 was increased significantly in DKD mice (Fig. 3A, B). Western blot results showed that the expression of CAV1 increased significantly under HG stimulation (Fig. 3C, D). After high-dose losartan intervebtion, the expression of CAV1 was decreased in hrGECs under HG stimulation (Fig. 3E, F). These findings suggest that losartan may inhibit HG-related kidney injury by regulating CAV1 expression.
HG up-regulated CAV1 expression and losartan inhibited this process. (A) Representative immunohistochemical (IHC) staining of CAV1 in kidney. (B) Statistical data of IHC staining of CAV1 (n≥5). ***P<0.001 vs. Control. (C) Representative western blot of CAV1; (D) Densitometric analysis of CAV1 western blot signals (n≥3). *P<0.05 vs. NG, **P<0.01 vs. NG. (E) Western blot of ANGPT2 of hrGECs incubated with NG, HG, HG+Los-L, HG+ Los-H. (F) Densitometric analysis of western blot signals of ANGPT2 (n≥3). *P<0.05 vs. NG group, #P<0.05 vs. HG group. Data are presented as means±SEM.
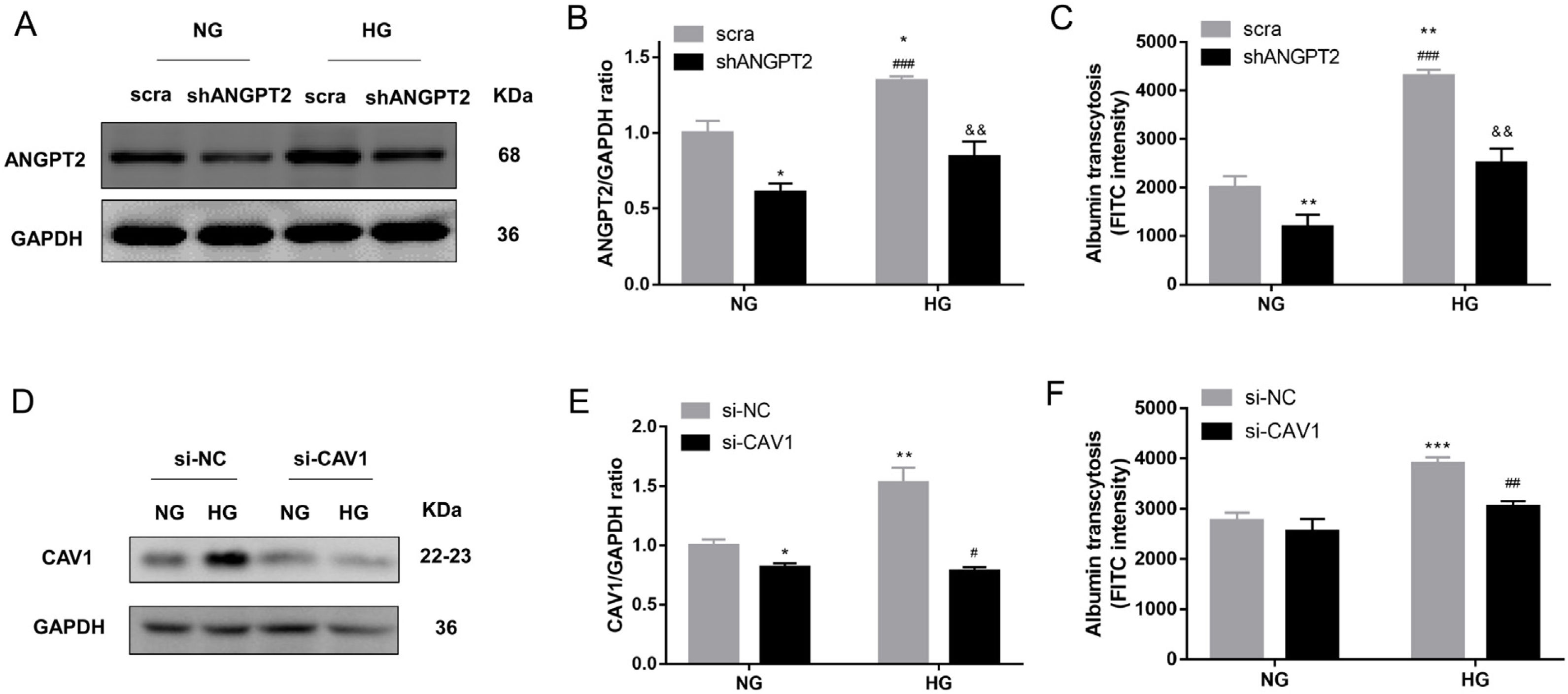
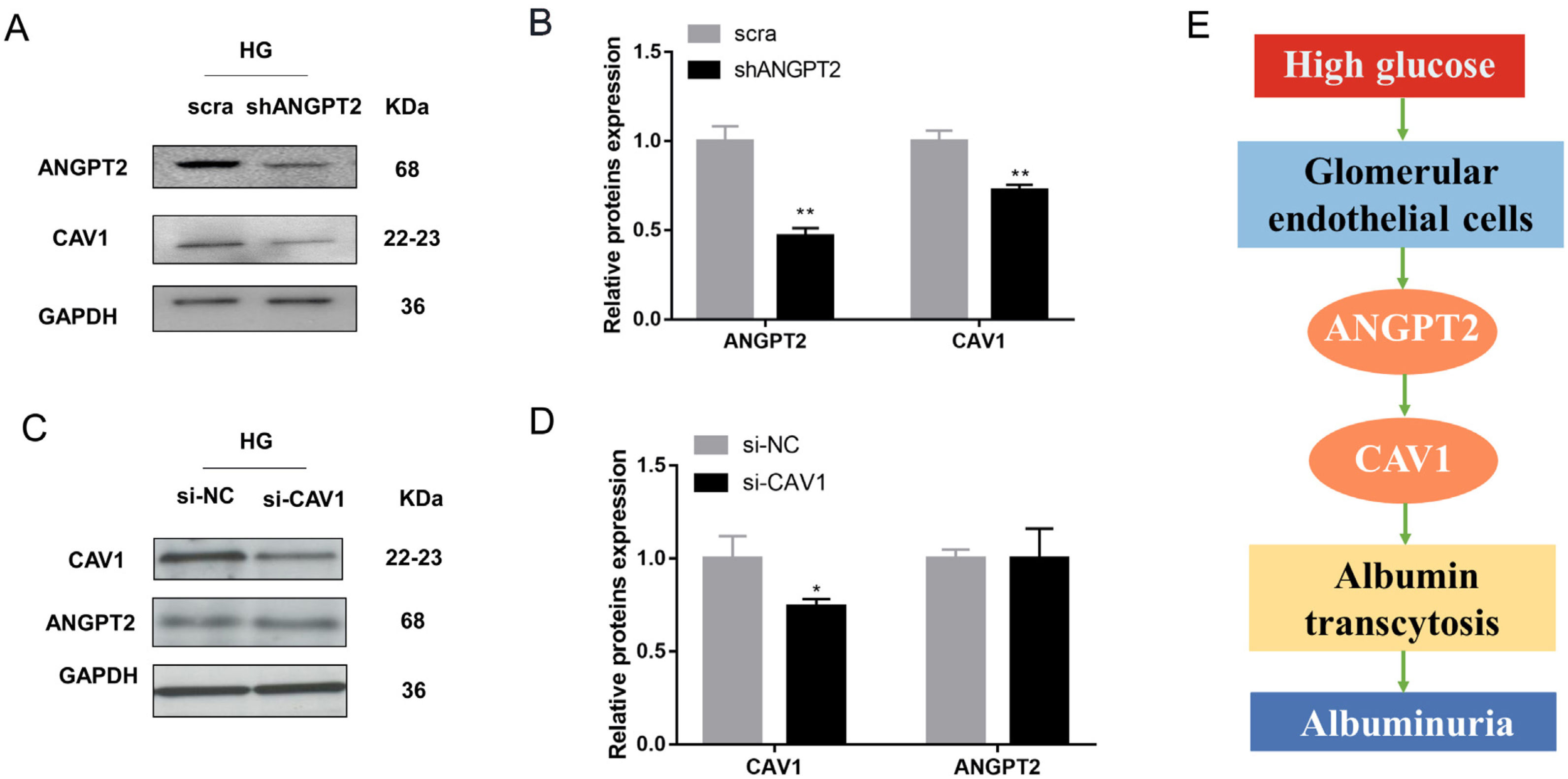
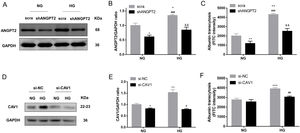
To identify the role of ANGPT2 in albumin transcytosis, hrGECs were transfected with lentiviruses expressing shRNA targeting ANGPT2 to specifically knock down ANGPT2 expression (Fig. 4A, B), which caused a decrease in albumin transcytosis both in NG and HG groups (Fig. 4C). To explore whether CAV1 is involved in HG-related kidney injury by regulating albumin transcytosis, hrGECs were transfected with siCAV1 to specifically knock down CAV1 expression in NG and HG conditions (Fig. 4D, E). Under HG condition, the decrease in CAV1 expression reduced albumin transcytosis in hrGECs. However, there was no significant effect of albumin transcytosis in the NG group (Fig. 4F). Thus, we concluded that ANGPT2 and CAV1 could promote albumin transcytosis of hrGECs during HG exposure.
ANGPT2 and CAV1 downregulation inhibited albumin transcytosis in hrGECs. (A) Representative western blot of ANGPT2 with shANGPT2 for 72h. (B) Densitometric analysis of ANGPT2 western blot signals (n≥3). *P<0.05 vs. NG+scra (scramble shRNA), ###P<0.001 vs. NG+shANGPT2, &&P<0.01 vs. HG+ scra. (C) FITC-albumin uptake analysis. **P<0.01 vs NG+ scra, ###P<0.001 vs. NG+shANGPT2, &&P<0.01 vs. HG+ scra. (D) Representative western blot of CAV1 with si-CAV1 for 48h. (E) Densitometric analysis of CAV1 western blot signals (n≥3). *P<0.05 vs. NG+si-NC, **P<0.01 vs. NG+si-NC, #P<0.05 vs. HG+si-NC. (F) FITC-albumin uptake analysis. ***P<0.001 vs NG+ scra, ###P<0.001 vs. HG+si-NC. Data are presented as means±SEM.
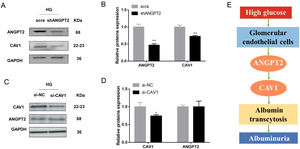
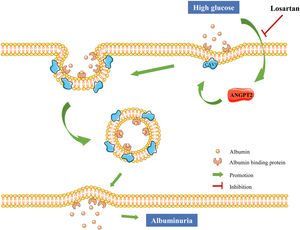
To further explore the relationship between ANGPT2 and CAV1 in hrGECs, we knocked down ANGPT2 to detect the expression of CAV1 under high glucose conditions. Upon transfection with shANGPT2 to specifically knock down ANGPT2, the expression of CAV1 was reduced (Fig. 5A). Statistical analysis confirmed this result (Fig. 5B). When we knocked down CAV1 in hrGECS under HG conditions, the expression level of ANGPT2 was not significantly changed (Fig. 5C, D). Thus, by summarizing the experimental results, we concluded that ANGPT2 could positively regulate the expression of CAV1. In summary, under HG stimulation, the expression of ANGPT2 in GECs increases, which leads to an increase in albumin transcytosis by promoting the expression of CAV1 and ultimately increasing the excretion of urinary albumin (Fig. 5E).
Regulation between ANGPT2 and CAV1 in hrGECs. (A) Western blot of ANGPT2 and CAV1 after transfection with shANGPT2 in HG condition. (B) Densitometric analysis of western blot signals of ANGPT2 and CAV1 (n≥3). **P<0.01 vs. scra. (C) Western blot of ANGPT2 and CAV1 after transfection with si-CAV1 in HG condition. (D) Densitometric analysis of western blot signals of ANGPT2 and CAV1 (n≥3). *P<0.05 vs. si-NC. (E) Summary chart of relationship between ANGPT2 and CAV1 in GECs during HG exposure. Data are presented as means±SEM.
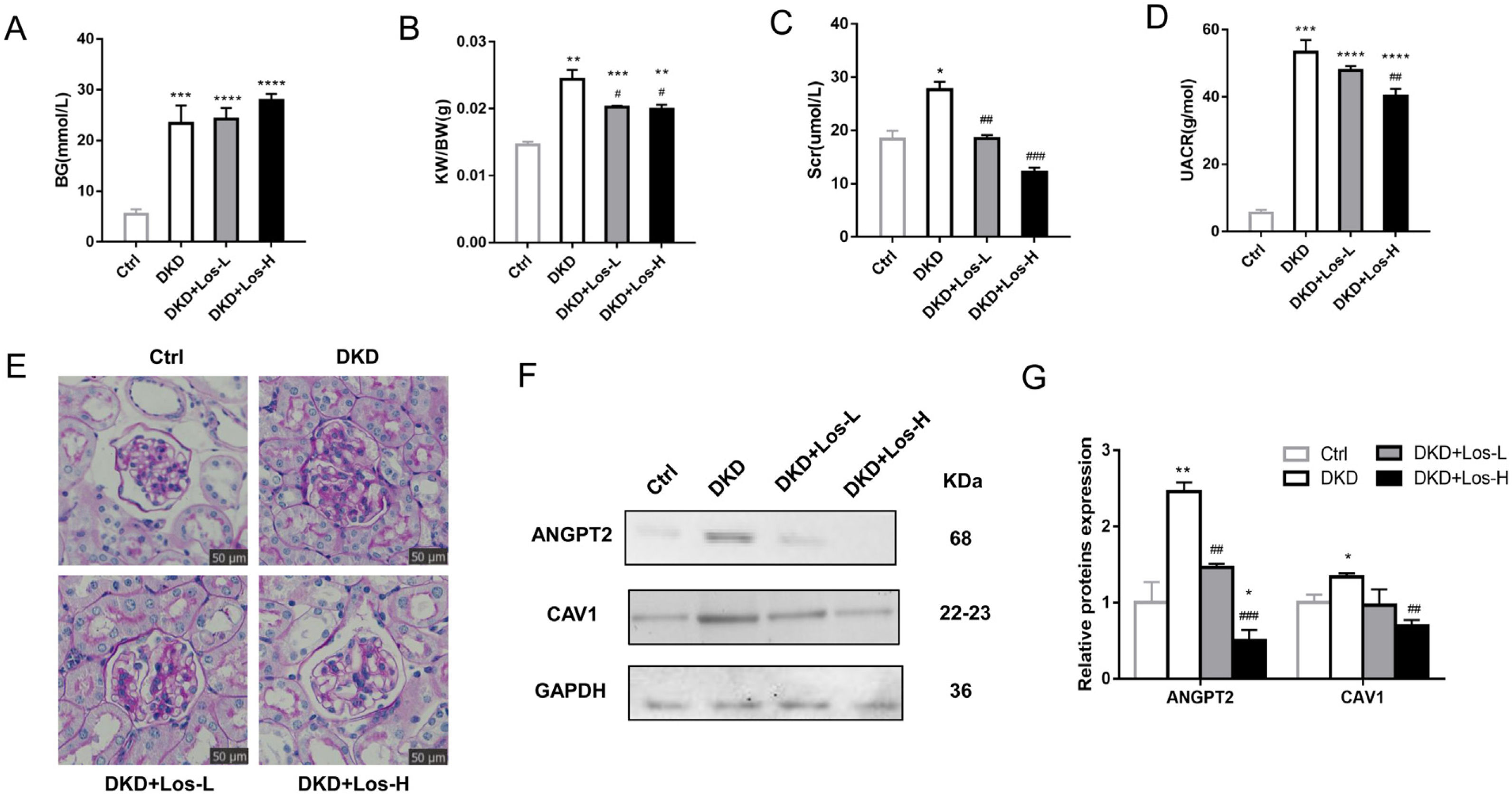
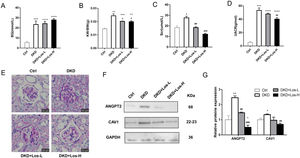
Compared with control mice, the levels of blood glucose (Fig. 6A), ratio of kidney weight/body weight (Fig. 6B), and urine albumin/creatinine ratio (Fig. 6D) were obviously higher in DKD mice treated with losartan. There were no detectable changes in the blood glucose of DKD mice treated with losartan compared with DKD mice (Fig. 6A). However, compared with DKD mice, mice administered losartan showed an improved ratio of kidney weight/body weight, serum creatinine and urine albumin/creatinine ratio (Fig. 6B–D). PAS staining revealed that glomerular and tubular basement membrane thickening, mesangial matrix accumulation, and mesangial expansion were reduced by losartan treatment in a dose-dependent manner (Fig. 6E).
Losartan inhibited albumin transcytosis by down-regulating ANGPT2 and CAV1 expression in vivo. (A) Blood glucose level (BG). (B) Ratio of the kidney weight/body weight (KBWR). (C) Serum creatinine (Scr). (D) Urine albumin/creatinine ratio (UACR). (E) Representative PAS (periodic acid-Schiff) staining images of kidney. (F) Western blot of ANGPT2 and CAV1 of kidney in Ctrl group, DKD group, DKD+ Los-L(10mg/kg/d) group and DKD+ Los-H(50mg/kg/d) group. (G) Densitometric analysis of western blot signals of ANGPT2 and CAV1 (n≥3). *P<0.05 vs. Ctrl group, #P<0.05 vs. DKD group. Data are presented as means±SEM.
Then, we examined the effects of losartan on the expression of ANGPT2 and CAV1. Western blot results showed that the expression of ANGPT2 and CAV1 was increased significantly in DKD mice, which was consistent with the previous results of immunohistochemistry staining. Compared with DKD mice, the expression of ANGPT2 and CAV1 was decreased by losartan treatment in a dose-dependent manner (Fig. 6F, G). Based on the above results, we concluded that losartan reduced albumin transcytosis in GECs during HG exposure by regulating ANGPT2 and CAV1 expression.
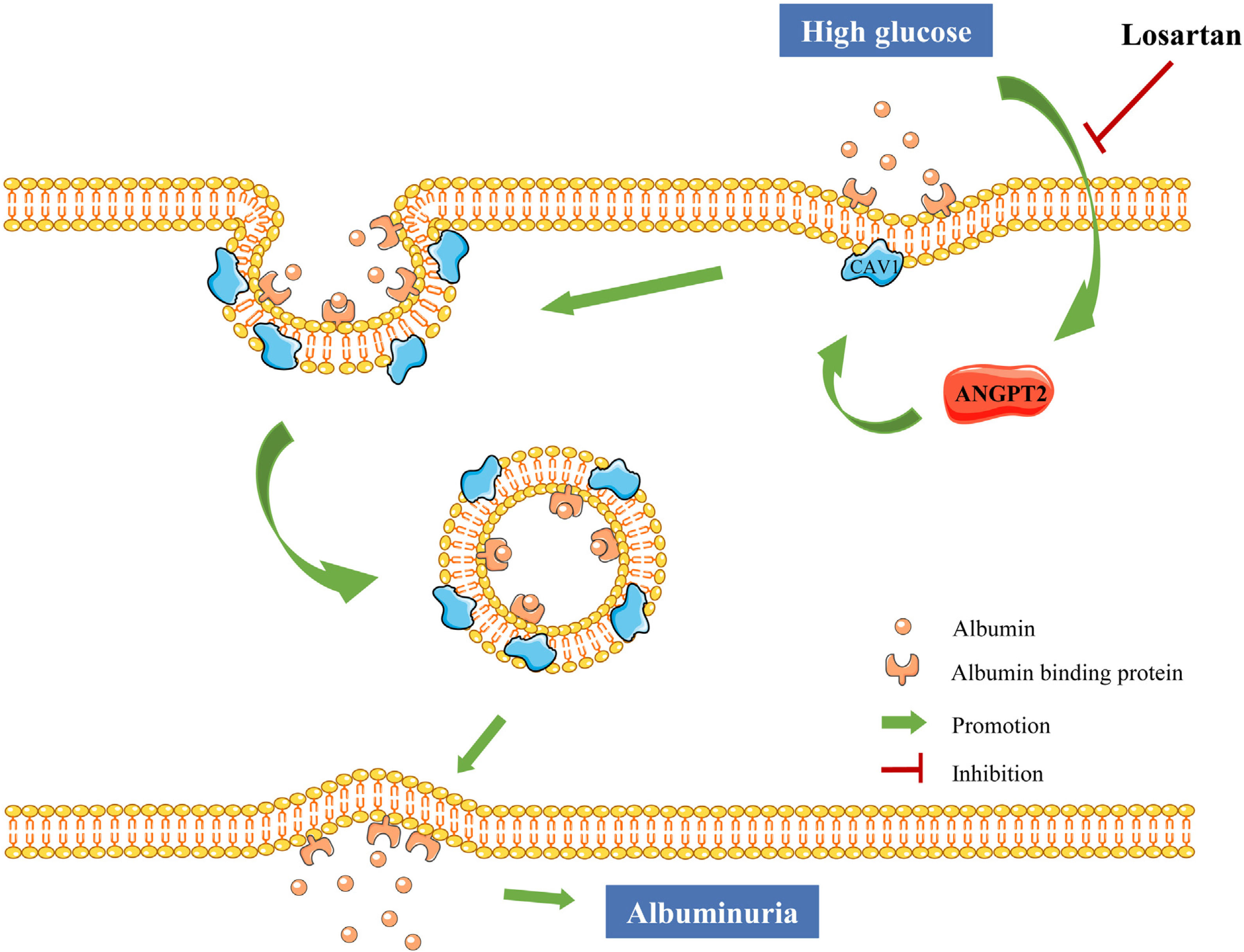
DiscussionAs the cells of the glomerulus are in direct contact with the blood, the transcytosis of albumin across GECs has been reported to be associated with albuminuria.11,35,36 However, the exact mechanism of pathological transcytosis leading to albuminuria in GECs remains unknown. Our data show that ANGPT2 increased the transcytosis of albumin across GECs under HG conditions by promoting CAV1 expression, thus increasing albuminuria. Losartan reduced albumin transcytosis of GECs by downregulating ANGPT2-CAV1 under HG conditions, thus reducing the production of albuminuria (Fig. 7).
The model of albumin transcytosis in vitro showed that albumin transcytosis across hrGECs was a concentration-dependent and time-dependent process. In addition, the transcytosis of albumin across hrGECs was significantly increased under HG conditions. These results are consistent with previous studies.11
In rats, ANGPT2 transcripts were detected in mature podocytes, which impair podocyte function by enhancing transendocytosis of plasma albumin, whereas in mice, ANGPT2 is only transiently expressed and almost undetectable in the mature glomeruli.37 The expression of ANGPT2 is upregulated in diabetic glomerulopathy and immune-mediated glomerulopathy.38 Consistent with previous studies, our immunohistochemical results confirmed that the expression of ANGPT2 was extremely low in the mature glomeruli of normal mice. Compared with that in normal mice, ANGPT2 expression was increased in the glomeruli of DKD mice. Moreover, our study shows that the expression of ANGPT2 was increased under HG stimulation and that ANGPT2 promotes albuminuria by enhancing the transcytosis of albumin in GECs.
It has been shown that Src kinase promotes albumin transcytosis across GECs by activating CAV1 phosphorylation.11 Our previous research found that the upregulation of ANGPT2 expression can inhibit albumin transcytosis across renal tubular epithelial cells by activating CAV1 phosphorylation during HG exposure.12 In this study, ANGPT2 regulated albumin transcytosis across GECs by regulating CAV1 expression rather than activating CAV1 phosphorylation during HG exposure. This difference may be due to the various regulatory mechanisms of CAV1 in different cell types. In addition, ANGPT2 is not a kinase and does not directly regulate CAV1 phosphorylation. The regulatory mechanism between ANGPT2 and CAV1 remains to be further explored.
The pathogenesis of albuminuria is complex and elusive. Combined with our previous studies, we provide some evidence for the important role and mechanism of albumin transcytosis across GECs and renal tubular epithelial cells in the formation of albuminuria. In GECs, ANGPT2 promotes albumin transcytosis under high glucose conditions by increasing CAV1 expression, thus increasing albumin excretion. In renal tubular epithelial cells, ANGPT2 inhibits albumin transcytosis during HG exposure by activating CAV1 phosphorylation, resulting in the decreased tubular reabsorption of albumin. These two mechanisms jointly participate in the formation of albuminuria in DKD. In addition to CAV1, ANGPT2 is another potential therapeutic target for albuminuria in DKD.
Many studies have suggested that losartan plays an important role in reducing albuminuria,39,40 but the mechanism has not been clarified. In vitro, we found that losartan reduced albumin transcytosis across hrGECs during HG exposure. In addition, losartan treatment reduced the expression of ANGPT2 and CAV1 in a dose-dependent manner after high glucose intervention. In vivo, the expression levels of ANGPT2 and CAV1 in the kidneys of DKD mice were both downregulated after losartan treatment. These results provide new evidence on the underlying mechanism of losartan in the development of DKD.
The major limitation of this study is that we only confirmed the regulatory relationship between ANGPT2 and CAV1 in vitro. In the future, we will further verify the regulation of ANGPT2 and CAV1 and the mechanism of losartan on albumin transcytosis in vivo. It is of great significance to explore the mechanism of albumin transcytosis therapy to search for novel therapeutic targets of diabetic albuminuria. Our study will provide more evidence for the study of the transcytosis of albumin in the kidney and provide a potential therapeutic target for the treatment of DKD albuminuria.
ConclusionIn summary, ANGPT2 promotes albumin transcytosis across GECs under HG conditions by increasing CAV1 expression, thus increasing albumin excretion. Losartan reduces albumin transcytosis and albuminuria formation in DKD by inhibiting the upregulation of ANGPT2 under HG conditions. Our research is the first to investigate the role and mechanism of ANGPT2 in albumin transcytosis across GECs and the inhibitory effect of losartan on this process. Our findings suggest that ANGPT2 and CAV1 are novel therapeutic targets for diabetic albuminuria. In addition, we provide new evidence on the mechanism of losartan in the development of DKD.
Authors’ contributionsWY and SH designed the study. ZD, GY and CY conducted the experiments. CY, LH and JH analyzed the data. CY and LH wrote and drafted the manuscript. All authors read, edited and approved the final manuscript.
Ethics approvalAll animal experiments were approved by the Institutional Animal Care and Use Committee at Tongji Medical College, Huazhong University of Science and Technology (No. 2019S2570).
Consent for publicationNot applicable.
Availability of data and materialsThe data generated over the course of the present study are available from the corresponding author upon request.
Conflict of interestThe authors declare they have no conflict of interest.
This work was supported by grants from the National Natural Science Foundation of China (No. 81570657, No. 81974111 and No. 30900682), Hubei Province Health & Family Planning Commission (No. WJ2015MB013).