Dear Editor,
Temsirolimus, a new inhibitor of the mammalian target of rapamycin (mTOR), has shown to prolong the survival rate of patients with advanced renal carcinoma.1We report the case of a man who suffered from acute renal failure (ARF) during his treatment with this drug.
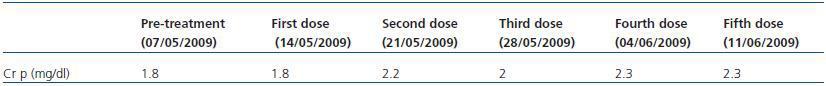
A 63-year-old male with important medical history of ischaemic heart disease, type 2 diabetes mellitus initially treated with gliclazide and hypertension treated with Carvedilol and Enalapril. In June 2008, he was diagnosed with left renal carcinoma with pulmonary metastasis, for which it was decided to carry out a left nephrectomy and chemotherapy. He was initially treated with Sunitinib from August to October 2008; afterwards, from November 2008 to March 2009, he received Sorafenib. Since May 2009, due to the progression of his illness, he began treatment with Temsirolimus at a dose of 25mg IV/week. The analytical progress of the pre-treatment and that following the administration of Temsirolimus is shown in Table 1.
Before his scheduled visit to receive the sixth dose of Temsirolimus, the patient mentioned fatigue, a decrease in the ingestion of liquids and solids (due to ulcers in the oral mucosa) and a drop in urine volume. He did not mention any other symptoms.
During the physical examination, the patient was in good general state, was conscious and had clarity of mind, had no temperature, his blood pressure was 90/50mmHg and had two ulcers in the oral mucosa. There were no other pathological findings in the rest of the examination.
The renal Doppler ultrasound showed a normal right kidney, with good vascularisation.
In detail, Creatinine of 6.5mg/dl, Sodium of 133mEq/l, Potassium of 5.1mEq/l, Uric acid of 11mg/dl, Calcium of 10.7mg/dl and Haemoglobin of 13.4g/dl were found in the blood, while the rest of the complete blood count and coagulation were within normal values. Urine analysis: Creatinine of 192mg/dl and Sodium of 69mEq/l. Protein quantification in the 24-hour urine was negative.
In the first 24 hours following admission, the renal function continued to change with a creatinine of 7.6mg/dl, despite adequate hydratation with a saline solution, later improving without needing renal substitutive treatment and with creatinine levels of 2.2mg/dl at discharge.
The treatment of advanced renal carcinoma with Temsirolimus was well-tolerated, and the majority of the side effects reported were medically controlled.2 Gerullis et al analysed the tolerance to Temsirolimus in 32 patients with advanced renal carcinoma, with a slight increase in creatinine present in 40.6% of them, without leading to ARF or the need for renal replacement therapy.3 From the start of the treatment, we also noticed in our patient a slight increase in the levels of plasma creatinine, contributing to the lack of ingestion of solids and liquids. This was linked with the consumption of angiotensin-converting enzyme inhibitors, to which the patient presented a reversible ARF. Although ARF is not described as a frequent side effect of Temsirolimus, its nephrotoxic potential could be when considering this slight increase in creatinine, which aggravated renal damage through other functional factors (volume depletion, NSAIDs, etc.), as in the case of our patient. Given the fact that the primary purpose of this drug is to treat advanced renal carcinoma, when treating patients who have undergone nephrectomy, thus with a greater susceptibility to renal deterioration, the possible nephrotoxicity of Temsirolimus is of a particular interest in these patients. It is advisable therefore to monitor their renal function.
Table 1. Progress of the renal function before and after treatment with Temsirolimus