INTRODUCTION
In the last issue of Nefrologia 2012;32(3):367-73,, del Pozo et al.1 reflected on the discrepancies encountered in the use of metformin in type 2 diabetes mellitus (DM2) patients: the lack of standardised criteria for indicating this drug in different stages of renal failure and its use in these patients.
We believe that this article deserves a very meticulous commentary in the form of an editorial regarding the importance of this issue in daily clinical practice.
GLYCAEMIC METABOLIC CONTROL IN CHRONIC KIDNEY DISEASE PATIENTS
In patients with type 2 DM that also suffer from chronic kidney disease (CKD), the best possible metabolic control is that which prevents and/or slows the progressive evolution of the disease, while also acting on other progression factors such as arterial hypertension, albuminuria, tobacco use, and obesity. The treatment objectives for glycaemic control tend to be expressed in terms of a target glycosylated haemoglobin values (HbA1c) <7%.2 In order to achieve this optimal control, two primary obstacles must be surpassed: the inconvenience of administering certain glucose-lowering drugs that are contraindicated in CKD, and the predisposition towards hypoglycaemia in these patients.
A) Oral anti-diabetic drugs
Classification of oral anti-diabetic drugs
Oral anti-diabetic drugs (OAD) are classified into:
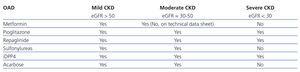
- Insulin secretagogue drugs (Table 1): sulfonylureas, meglitinides, dipeptidyl-peptidase 4 inhibitors (iDPP4).
- Drugs that stimulate the peripheral activity of insulin: metformin, pioglitazone.
- Drugs that inhibit α-glycosidase in the intestines (delay the absorption of glucose): acarbose, miglitol.
General characteristics of the primary groups of oral anti-diabetic drugs
Sulfonylureas: These drugs are capable of stimulating β-cells in order to increase endogenous secretion of insulin. Sulfonylureas are contraindicated in patients with renal failure. Their main secondary side effects include hypoglycaemia, which can be severe, and weight gain.
Meglitinides (repaglinide and nateglinide): Both of these drugs have a short half-life, and so are administered before each meal. Repaglinide is more potent, and is primarily eliminated in the bile, making its use acceptable in any stage of CKD, even in patients on dialysis. Nateglinide, despite being metabolised in the liver, is degraded into active metabolites that are filtered out in the kidneys, making this drug unadvisable in patients with CKD. Both drugs can produce hypoglycaemia, although this condition is produced less frequently than with sulfonylureas because of the shorter half-lives.
Metformin: Metformin is a biguanide whose primary mechanism of action is reducing the level of glucose production in the liver by acting on gluconeogenesis, although it also increases muscle uptake of glucose. It is eliminated by the kidneys, and so the technical data sheet contraindicates its use in patients with creatinine clearance <60ml/min due to the risk of lactic acidosis. In fact, it can be used up to a glomerular filtration rate (GFR) of 30ml/min/1.73m2 (see explanation below).
Glitazones: Rosiglitazone was removed from the market due to the possibility of increased cardiovascular risk. Its primary mechanism of action is to increase the body’s sensitivity to insulin by increasing the uptake and use of glucose in muscle and fat tissue. It also decreases, to a lesser degree, gluconeogenesis and the synthesis of fatty acids in the liver. It is metabolised in the liver and excreted in faeces, and so can be used in any stage of CKD. Its association with weight gain, which is due to water retention, has led to the contraindication against its use in patients with heart or liver failure, and the possibility of distal fractures in women must also be taken into account. In France and Germany, pioglitazone has been removed from the market due to the possibility of increased risk of bladder cancer.
α-Glycosidase inhibitors (acarbose and miglitol): Produce a competitive and reversible inhibition of α-glycosidase in the intestinal microvilli, delaying the absorption of complex carbohydrates and decreasing the post-prandial glycaemic peak. In monotherapy, these drugs do not increase weight or hypoglycaemia. They are contraindicated in severe CKD.
Dipeptidyl-peptidase 4 inhibitors: These drugs intensify the activity of incretin by inhibiting the enzyme dipeptidyl-peptidase IV, which degrades glucagon-like peptide 1 (GLP-1), which is produced in the intestines in response to eating. GLP-1 stimulates the secretion of insulin and inhibits the secretion of glucagon. In this manner, these drugs produce a physiological secretion of insulin mediated by food intake, and an inhibition of excessive glucagon. The iDPP4 that have been commercialised in Spain currently include: sitagliptin, vildagliptin, saxagliptin, and linagliptin. This group of drugs provides major advantages over classically used secretagogues, since they do not produce hypoglycaemia, by using an insulin secretion stimulation mechanism that is not dependent on glucose levels, a characteristic that makes these drugs especially attractive in patients with CKD due to the predisposition of these patients to developing hypoglycaemia.
Sitagliptin, vildagliptin, and saxagliptin require dosage adjustments, since the elimination of these drugs is essentially through the kidneys. Recent studies with linagliptin have shown that this drug is eliminated by the liver and bile, and that its half-life extends to a maximum of 14-18 hours in patients with advanced CKD, obviating the need for dosage adjustments. This drug has even been used in diabetic patients on haemodialysis.
Oral anti-diabetic drugs in patients with chronic kidney disease who are not on dialysis
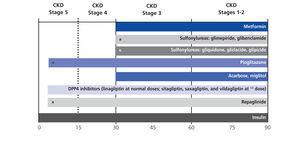
Table 2 summarises the indications for the different therapeutic groups of oral anti-diabetic drugs for use in chronic kidney disease, and the Figure displays the usage of these different therapeutic groups of oral anti-diabetic drugs and insulin therapy in the different stages of chronic kidney disease.
For proper management of hyperglycaemia, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD), in 2008,3 recommended that all patients with type 2 DM be treated with metformin from the moment of diagnosis, except when formally contraindicated or not tolerated by the patient. Initial treatment with metformin continues to be the recommendation in updated guidelines from both societies3,4 and this protocol has also been backed by the major Spanish medical societies involved in the treatment of type 2 DM.5 However, the technical data sheet for metformin displays a contraindication for using the drug when creatinine clearance <60ml/min. The NICE guidelines6 and recent studies7 indicate that it can be safely used in patients with creatinine clearance >30ml/min, recommending a dosage reduction when glomerular filtration rate (GFR) <45ml/min/1.73m2. The Food and Drug Administration (FDA) bases its recommendations solely on creatinine concentrations (contraindicating the use of metformin when serum creatinine ≥1.5mg/dl in males and ≥1.4mg/dl in females). Recently, a study was published in which metformin was used in patients with creatinine clearance of approximately 20ml/min, with doses of 200-500mg/day.8 As Lipska et al. suggested,9 it would appear reasonable to suspend the use of metformin in patients with an estimated GFR (eGFR) <30ml/min/1.73m2, or a GFR<45ml/min/1.73m2 in patients with risk factors for developing lactic acidosis (peripheral hypoperfusion, diabetic foot, heart failure, advanced liver disease, or a previous history of lactic acidosis).
Changes to treatment protocols must be made early in order to prevent or delay complications, instating combined treatment regimens early. The technical data sheets for sulfonylurea are contraindicated for patients with severe renal failure (stage 4 or 5 CKD). In stage 3 CKD, gliquidone, glipizide, and gliclazide can be used, although there is a high risk of hypoglycaemia.
Repaglinide is eliminated primarily through the bile, and only 8% is eliminated by the kidneys, making this drug a feasible option in patients with any stage of CKD.10 However, it is contraindicated when patients are taking gemfibrozil, due to the possibility of an increased risk of hypoglycaemia if the repaglinide dose is not adjusted. In contrast, nateglinide is metabolised by the liver, forming various active metabolites that are eliminated through the kidneys. Metabolisation of this drug through the CYP2C9 may provoke interactions with several different commonly used drugs (amiodarone, warfarin). Cases of severe hypoglycaemia have been described when administering this drug in patients with kidney disease.11 This makes it unadvisable to administer this drug in patients with CKD.
Few studies have examined the use of iDDP4 in patients with type 2 DM and altered renal function, with a very small number of patients in the case of sitagliptin,12,13 saxagliptin,14 and vildagliptin.15 Sitagliptin, vildagliptin, and saxagliptin require dosage adjustments when creatinine clearance falls below 50ml/min, since these drugs are primarily eliminated by the kidneys. This form of sitagliptin (50mg) is not currently commercially available in Spain. Half doses of saxagliptin (2.5mg) are commercially available, but are not currently subsidised in Spain. One recent study involving 124 patients with type 2 DM and severe CKD (mean eGFR: 21.9±5.7ml/min/ml/min/1.73m2) showed that vildagliptin effectively decreased HbA1c levels, and adverse effects were similar to those produced in the placebo group.15 In the case of vildagliptin, the recommended dose in patients with CKD on dialysis is 50mg/day (1 pill), instead of every 12 hours. Linagliptin deserves special mention for being the only iDPP4 that is almost exclusively eliminated by the bile, allowing for its use in patients with any stage of CKD, including those on dialysis, without requiring an adjustment of the dosage (Table 3).16
The group of α-glycosidase inhibitors has limited hypoglycaemic activity. Acarbose is not absorbed for the most part. Miglitol is well absorbed, but not metabolised, and is eliminated by the kidneys with a half-life of 2-3 hours. Plasma concentrations increase in patients with renal failure, and so miglitol is contraindicated in patients with CKD.
Pioglitazone can be administered in patients with any stage of CKD, although in severe CKD, one must take precautions due to the possibility of adverse effects (weight gain, oedema, and worsening of heart failure). This drug does not require dosage adjustments in mild or moderate CKD. In patients with CKD, plasma concentrations of pioglitazone and its metabolites are lower than in individuals with normal renal function. However, clearance of the original substance is similar. As such, the concentration of free pioglitazone remains unaltered, and so its use is not contraindicated in patients with CKD. Pioglitazone can produce fluid retention in mild or moderate cases of CKD, and so this condition should be more closely monitored in patients that might have heart failure. Proper diuretic treatment must also be applied when this drug is used. Pioglitazone is indicated in combined therapy with sulfonylureas, metformin, iDPP4, or insulin. In monotherapy, it is only indicated if treatment with metformin is contraindicated (in the case of mild or severe CKD) or when patients are intolerant to metformin.
In the case of patients with CKD and creatinine clearance <30-60ml/min, a combination of iDPP4 or repaglinide (which improve the secretion of insulin in the case of persistent insulin reserve) with pioglitazone (which improves sensitivity to insulin), can be used only if the patient is not prone to fluid retention. iDPP4 agents present a major advantage over repaglinide by not producing hypoglycaemia.
Oral anti-diabetic drugs in patients with chronic kidney disease on dialysis
As GFR decreases, insulin is broken down to a lesser degree. In this situation, patients perceive that they require a lower dose of OAD (which may even be temporarily suspended due to lengthened lifetime of endogenous insulin) or insulin. As such, patients frequently refuse insulin treatment when taking OAD. When receiving insulin, patients may perceive an improvement in the treatment of hyperglycaemia.
Sulfonylureas should be avoided in patients on haemodialysis. They bind strongly to albumin, and so high levels of the drug cannot be eliminated through haemodialysis. Simultaneous administration of beta-blockers, aspirin, or dicoumarin increases the proportion of free drug molecules in the blood and can produce severe hypoglycaemia. This situation is less severe with certain sulfonylureas (glipizide and glimepiride). However, there are not recommended in a patients on haemodialysis.
Glitazones are associated with a high risk of oedema and heart failure, which increases as GFR decreases. As such, their use is not recommended in patients on dialysis, although they can be used in patients with advanced CKD. Since repaglinide is metabolised in the liver, it can be used in these patients, although with strict monitoring in light of the high risk of hypoglycaemia. Treatment must be started with a minimal dosage (0.5mg) and subsequently, dosage must be carefully monitored. Recently, positive results were published from a study in which hyperglycaemia was treated with vildagliptin in patients with type 2 DM on haemodialysis, with no secondary side effects or hypoglycaemia produced.17 Linagliptin can be used in dialysis patients because it is almost exclusively eliminated by the bile. However, insulin treatment continues to be the treatment of choice in patients on dialysis, both haemodialysis and peritoneal dialysis. Undoubtedly, further research into the management of new iDPP4 in patients with advanced CKD or on dialysis will provide us with new perspectives for managing hyperglycaemia in these patients.
B) Insulin therapy
Dosage adjustments for insulin in patients with chronic kidney disease not on dialysis
Chronic renal failure is associated with a decrease in renal catabolism of insulin. As such, glycaemia levels in diabetic patients with renal failure on treatment with insulin must be closely monitored and adjusted for personalised treatment. However, certain general guidelines have been established for dosing insulin treatment in these patients18,19:
- Dosage does not require adjustments if GFR is >50ml/min/1.73m2.
- The dose of insulin should be reduced by 25% when GFR is between 10ml/min/1.73m2 and 50ml/min/1.73m2.
- The dose of insulin should be reduced by 50% when GFR is <10ml/min/1.73m2.
Subcutaneous insulin regimens in patients on dialysis
In haemodialysis, as in diabetic patients without renal failure, several different regimens of insulin therapy can be used, such as premixed insulin 2-3 times per day or baseline bolus prescriptions (slow-acting insulin along with fast-acting insulin before meals).20,21 Although there is no single regimen recommended for these patients,19 insulin analogues are preferable over human insulin, since analogues have shown to produce hypoglycaemia at a lower rate. As such, basal analogues (glargine, 1 per day, or detemir, 1-2 times per day) are preferred over NPH insulin (neutral protamine hagedom), and fast-acting analogues (lispro, aspart, and glulisine) are preferred over regular insulin (Table 4).
Intraperitoneal insulin in patients on peritoneal dialysis
In diabetic patients, peritoneal dialysis fluid solutions are recommended to have low glucose content or none at all, such as those based on amino acids or glucose polymers. Insulin can be administered subcutaneously or directly into the peritoneum, by introducing insulin into peritoneal dialysis boluses just before infusion into the peritoneum. If only the traditional subcutaneous route is used, 2 different doses of premixed insulin must be administered, 1 or 2 doses of slow-action insulin analogues, or even 3 doses of regular insulin together with meals in selected patients. The intraperitoneal approach provides both advantages and disadvantages over the subcutaneous approach, but allows for controlling glycaemia, and so may be the best option for the treatment of very disciplined, self-sufficient patients with motivation for close control of treatment. This administration route is more physiological than the subcutaneous approach, since insulin is absorbed directly in the portal vein as occurs in non-diabetic patients with endogenous insulin, reducing the secondary side effects associated with direct absorption of insulin into systemic circulation. Fast-acting insulin should always be used, and boluses should be administered just prior to infusion, matching up changes of the bolus with major meals. This method requires relatively large needles (3.8cm) in order to ensure the injection of the entire dose of the bolus into the dialysate. This bolus must be inverted on several occasions following injection in order to mix the solution properly.
In the case of automated peritoneal dialysis techniques (continuous cyclic peritoneal dialysis or nocturnal intermittent peritoneal dialysis), in which most of the dialysis sessions are performed at night, intra-peritoneal doses must be added as well as regular insulin whenever patients self-apply dialysis using a cycler, and this administration provides baseline coverage during the night.22-24
C) GLP-1 analogues
GLP-1 agonists bind to the receptors of this hormone, produced in the intestine before the arrival of the food bolus, increasing the secretion of insulin in the pancreas and inhibiting the secretion of glucagon. In addition, they slow gastric emptying and decrease appetite, therefore its use is associated with weight loss. The most common adverse effects of these drugs are nausea and vomiting. The two currently commercially available GLP-1 antagonists for treating type 2 DM are exenatid and liraglutid (Table 5).
Since it is eliminated by the kidneys, exenatid requires dosage adjustments when clearance is 30-50ml/min, and its use is not recommended in patients with stage 4 and 5 CKD (creatinine clearance <30ml/min).
The safety of liraglutid is not well established in patients with CKD, although pharmacokinetic analyses suggest that drug levels in the body are no different in this sub-group.25 Dosage adjustments for liraglutid are not required in mild CKD (clearance >60ml/min). Below this level, the extremely scarce experience in stage 3 cases and the lack of experience with patients with stage 4 and 5 contraindicate its use. Currently, these drugs cannot be recommended for use in patients with moderate or severe CKD, including patients with stage 5 CKD.
In conclusion, the current management of hypoglycaemic drugs in diabetic patients requires several conceptual modifications, which involves all health care professionals in charge of these patients. When a patient suffers from CKD, we must take into account not only the technical data sheet for anti-diabetic drugs, but also the metabolic pathways of these drugs and their safety profiles. Given the scarcity of studies carried out in patients with CKD that focus on treating hyperglycaemia, the ongoing development of new treatment options, and the high prevalence of diabetes mellitus in these patients, we must prioritise keeping our treatment protocols for hyperglycaemia in patients with CKD constantly updated.
Conflicts of interest
The authors have no conflicts of interest to declare.
Table 1. Oral anti-diabetic drugs and their use in chronic kidney disease according to drug technical data sheets
Table 2. Indications for the different treatment groups of oral anti-diabetic drugs in chronic kidney disease patients
Table 3. Approved uses of DPP4 inhibitors in renal failure and liver failure (2012)
Table 4. Pharmacokinetic characteristics of the different insulin compounds available
Table 5. Glucagon-like peptide 1 analogues in patients with chronic kidney disease
Figure 1. The use of different treatment options involving oral anti-diabetic drugs and insulin in the different stages of chronic kidney disease