Although the results of kidney transplantation (KT) have improved substantially in recent years, a chronic and inexorable loss of grafts mainly due to the death of the patient and chronic dysfunction of the KT, continues to be observed. The objectives, thus, to optimize this situation in the next decade are fundamentally focused on minimizing the rate of kidney graft loss, improving patient survival, increasing the rate of organ procurement and its distribution, promoting research and training in health professionals and the development of scientific registries providing clinical and reliable information that allow us to optimize our clinical practice in the field of KT. With this perspective, this review will deep into: (1) strategies to avoid chronic dysfunction and graft loss in the medium and long term; (2) to prolong patient survival; (3) strategies to increase the donation, maintenance and allocation of organs; (4) promote clinical and basic research and training activity in KT; and (5) the analysis of the results in KT by optimizing and merging scientific registries.
Aunque los resultados del trasplante renal (TR) han mejorado sustancialmente en los últimos años, continúa observándose una pérdida crónica e inexorable de los injertos debido principalmente a la muerte del paciente y a la disfunción crónica del TR. Por tanto, los objetivos para optimizar esta situación en la próxima década se centran fundamentalmente en minimizar la tasa de pérdida de injertos renales, mejorar la supervivencia de los pacientes, incrementar la tasa de obtención de órganos y su distribución, fomentar la investigación y la formación de los profesionales sanitarios y la elaboración de registros científicos que proporcionen una información clínica y fiable que nos permita optimizar nuestra práctica clínica en el campo del TR. Con esta perspectiva, esta revisión profundizará en: 1) estrategias para evitar la disfunción crónica y la pérdida del injerto a medio y largo plazo; 2) prolongar la supervivencia del paciente; 3) estrategias para incrementar la donación, mantenimiento y distribución de órganos; 4) promocionar la investigación clínica y básica y la actividad formativa en TR; y 5) el análisis de los resultados en TR mediante la optimización y fusión de los registros.
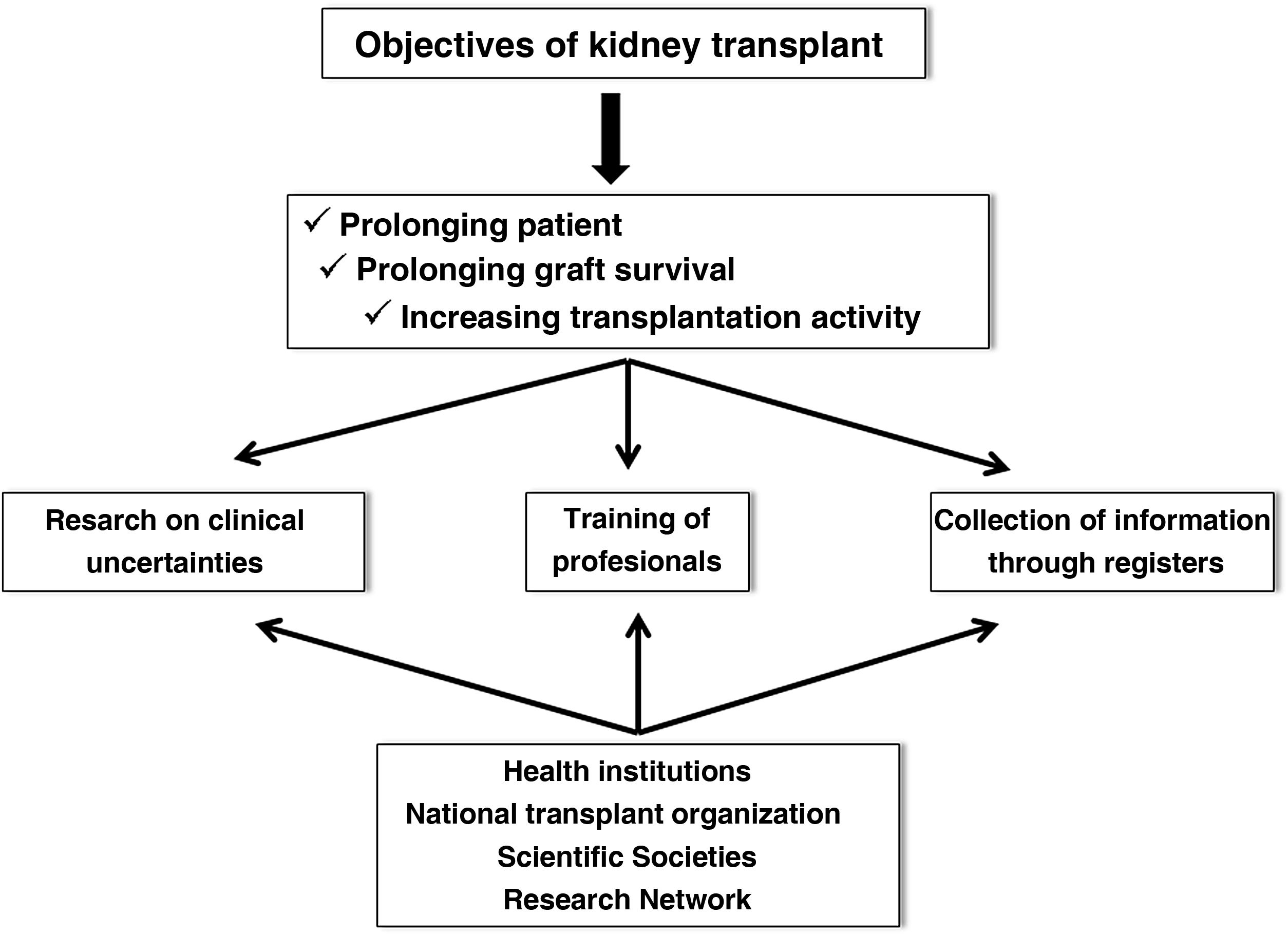
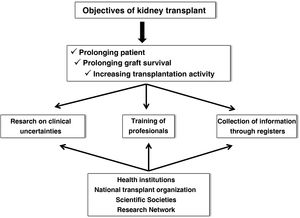
Renal transplantation (KT) represents the best therapeutic alternative in terms of survival for patients with advanced chronic kidney disease,1,2 provided that patients who are candidates for KT are adequately selected. Although the current results of KT have improved markedly with respect to previous times, chronic and inexorable graft loss continues to be observed even in the best starting conditions, such as living donor KT, and the premature death of many patients with respect to the general population of similar age and sex despite the optimization of renal function.3,4 Therefore, some of the most demanding goals in the field of KT for the next decade focus on prolonging graft and patient survival and increasing transplantation activity, improving access and distribution of organs. Undoubtedly, these objectives must be achieved through research into clinical uncertainties in the field of KT, good training of the healthcare personnel who care for these patients, and the collection and analysis of clinical and epidemiological information through registries. Obviously, this is not only achieved with an excellent attitude and aptitude of the professionals in this field, but must also be supported by health institutions, scientific societies, the National Transplant Organization (ONT) and research structures and networks (Fig. 1).
A priori, daily clinical practice shows an unfavorable clinical scenario in the field of KT. Independently of the high patient mortality, other factors such as the gradual increase in elderly donors, the early generation of donor-specific antibodies (DSA), the increasingly frequent subclinical immune dysfunction, the growing donation in asystole or accelerated graft senescence, could be some of the challenges that will have to be addressed by multidisciplinary strategies over the next decade with the intention of improving the results of RT. Therefore, to improve KT outcomes in the coming years, we should focus on the following aspects: 1) strategies to avoid chronic dysfunction and graft loss in the medium and long term; 2) prolong patient survival; 3) strategies to increase organ donation, maintenance and distribution; 4) promotion of clinical and basic research and training in RT; and 5) analysis of KT outcomes by optimizing and merging registries.
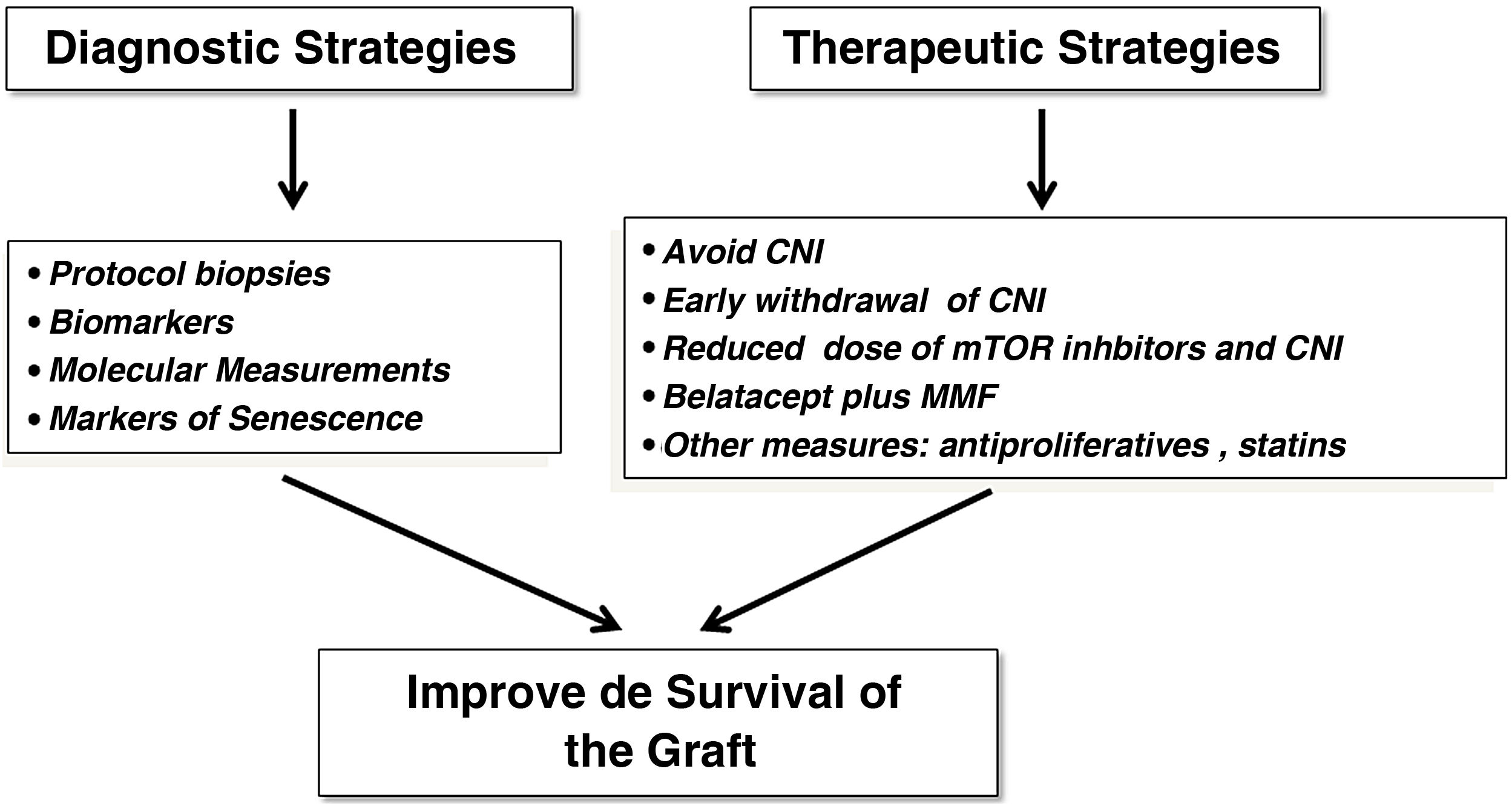
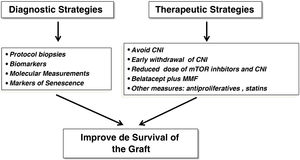
Avoid chronic dysfunction and graft lossWe have made substantial progress in the knowledge of some entities that potentially lead to chronic graft loss, such as acute rejection, but we still do not know exactly whether chronic graft dysfunction (CGD) per se, which is the second cause of graft loss after patient death, represents its own entity or is the product of premature graft aging secondary to multiple factors that damage the graft after implantation. Therefore, past and emerging diagnostic and therapeutic strategies could have a positive impact on renal graft survival (Fig. 2).
In this line, the systematic implementation of protocol biopsies (PB) of the graft, the search for new serological and molecular biomarkers of CGD the identification of senescence markers, the diagnosis of recurrence of primary renal disease and the early detection of BK virus infection, as well as the application of prognostic indices, could facilitate clinical decision-making and the development of therapeutic strategies in order to improve the results of KT in terms of survival.
The PB have demonstrated that subclinical inflammation is very frequent (30%–40%) after RT, even in patients at low immunological risk, and this lesion combined with interstitial fibrosis and tubular atrophy could impair graft function and survival at the longer term.5–8 However, other authors, using appropriate propensity models, have observed that graft survival after eight years of follow-up was similar in patients who underwent PB versus those who did not undergo this procedure.9 Therefore, controlled clinical trials are needed to clarify the clinical benefit of PB and the treatment of subclinical inflammatory lesions, especially borderline lesions, detected by PB. A multicenter clinical trial (Clinicaltrials.gov: NCT04936282) is currently underway to determine whether treatment of borderline inflammatory lesions with antilymphocyte globulin can reduce the occurrence of chronic lesions and graft loss in the medium term. If a strong benefit in graft survival is demonstrated with the detection and treatment of these subclinical inflammatory lesions, early PB (three to six months) should be implemented in the daily clinical practice of KT in the next decade. Likewise, the detection of inflammatory lesions with PB could also be important in patients at high risk of post-KT immune dysfunction (pre-KT hypersensitized, immune loss of previous graft, etc.) or when the clinical situation requires it for the sake of close immune surveillance. These aspects would undoubtedly clear doubts about the real performance of PB in the field of KT.
The PB are invasive techniques that are not risk-free and not all groups perform them. Therefore, in the coming years we urgently need the implementation of biomarkers for the early and non aggressive detection of immunological alterations or early renal graft dysfunction in order to carry out targeted therapies that improve graft survival.10,11 Indeed, a key point in the management of patients with KT is the early identification of graft damage before it becomes irreversible through the implementation of biomarkers in clinical practice. Although serum creatinine and impaired glomerular filtration rate (GFR) have been the most widely used biomarkers (to which we owe much of the success of RT), there is now a mass of scientific evidence on the usefulness of biomarkers from serum, tissue, cells, urine, that through the study of “omics” (genomics, transcriptomics, proteomics, metabolomics) could allow us to predict the evolution of the grafts, to know the pathogenic mechanisms involved in CGD and to personalize immunosuppression. Table 1 shows some of the biomarkers that have been used in different phases of KT to predict delayed renal function, graft tolerance, ischemia-reperfusion injury, or graft immune dysfunction. Although many of them are not routinely used in clinical practice, their implementation in the next decade could undoubtedly improve the results of KT and bring us closer to the exercise of precision medicine to make the best clinical decisions in this field.
Some of the noninvasive biomarkers analyzed in renal transplantation.
| Pretransplant | Clinical application | Clinical implementation |
|---|---|---|
| Urinary biomarkers derived from renal graft perfusion (e.g. NGAL, acetate, KIM-1, etc.) | Predict delayed renal function and the severity of ischemia-reperfusion injury. | Not yet systematically implemented in the clinic. More scientific evidence of their usefulness is needed |
| Extracellular vesicles | ||
| Post-KT rejection risk stratification | T-cell ELISPOT | Scientific evidence, but only implemented in some KT centers. |
| Cell-free DNA (cfDNA) | Ischemia-reperfusion damage | Not routinely used |
| Post-transplant | Clinical application | Clinical implementation | |
|---|---|---|---|
| Plasma creatinine and impaired GFR, determination of GFR by Iohexol. | Graft survival, graft rejection | Applicable in all KT centers | |
| Markers derived from the activity of the immune system: | |||
| Urinary chemokines: CXCL9, CXL10, CXCL13 and granzyme | Predictors of acute rejection and CGD | Not routinely used in clinical practice | |
| NGAL (neutrophil gelatinase-associated lipoprotein) from serum | Delayed renal function | Not routinely implemented in daily practice | |
| CD30 soluble | Acute rejection | Not routinely used | |
| Lymphocyte subpopulations (CD14, CD16, monocytes) in blood and aspirative cytology of the graft. | T-cell mediated acute rejection | Not routinely employed. Only implemented in some KT centers | |
| Detection of anti-HLA antibodies by the Luminex® technique. | Predictor of acute antibody-mediated rejection. | Routinely used in daily clinical practice | |
| Detection of HLA-DR/DQ molecular mismatches (eplet) | |||
| Urine transcriptomics: CD3 genesε mRNA, CXCL10 mRNA and 18ᴤRNA; circulating microRNA (miR-223-3p, miR-424-3p, miR-145-5p and miRNA-148a). | T-cell-mediated and antibody-mediated acute rejection. CGD (miR-148a) | Not routinely used | |
| Urinary proteomics and metabolomics (e.g., CKD273) | |||
| Cell-free DNA (cfDNA) | Predictor of acute rejection, DSA, BK virus nephropathy and CNI toxicity. | Not routinely used | |
| Serum, urinary and tissue Klotho | Renal graft senescence | Not routinely used | |
DNA: deoxyribonucleic acid; DSA: donor-specific antibodies; GFR: glomerular filtration rate; HLA: human leukocyte antigens; DNA: deoxyribonucleic acid; CGD: chronic graft dysfunction; HLA: human leukocyte antigens; CNI: calcineurin inhibitor drugs; KT: renal transplantation.
Recurrence of primary disease in the graft is another major current challenge in TX, accounting for up to one third of graft losses.12 Many glomerulonephritis considered de novo post-TX are in fact recurrences of primary glomerular disease, and the exact pathogenic mechanisms leading to this recurrence are not known, nor are the risk factors that condition such an unfavorable evolution. In this line, the paradigm is focal segmental hyalinosis (FSH), an entity that frequently recurs after TX (30%–50%), especially in young patients who initially debut with massive proteinuria, and that could be generated by mutations (30%) in specific genes (NPHS1, NPHS2, WT1) that affect glomerular permeability.13,14 Some urinary markers of recurrence of this entity have aroused interest in recent years, such as modified Apo A1b. The presence of this protein in urine has been observed in 93% of patients who suffered a post-TX recurrence of FSGH, whereas this was only observed in 9% of those with FSGH who did not suffer a recurrence. This confers to this protein a high sensitivity and specificity (93.4% and 91%, respectively) for the diagnosis of FSGH recurrence. This surprising finding opens a door to hope in the post-TX management of this disease.15,16 At the same time, it should make us reflect on the convenience of enhancing genetic diagnosis in nephrology units.
Early diagnosis of BK virus infection may allow the reduction of immunosuppression and improvement of this entity in a high percentage of patients. The use of low doses of anti-mTOR drugs and calcineurin inhibitors (CNI) in controlled clinical trials has demonstrated a lower incidence of BK infection without reducing the immunosuppressive potency.17 This strategy could be extended to those patients who receive a second KT after the loss of the first graft due to this infection. Apart from reducing immunosuppression, there is no effective therapy against this infection. The use of brincidofovir has been shown to be effective in reducing viral replication in human epithelial cells.18 Therefore, this drug could be a future alternative for these patients together with parenteral administration of immunoglobulins.
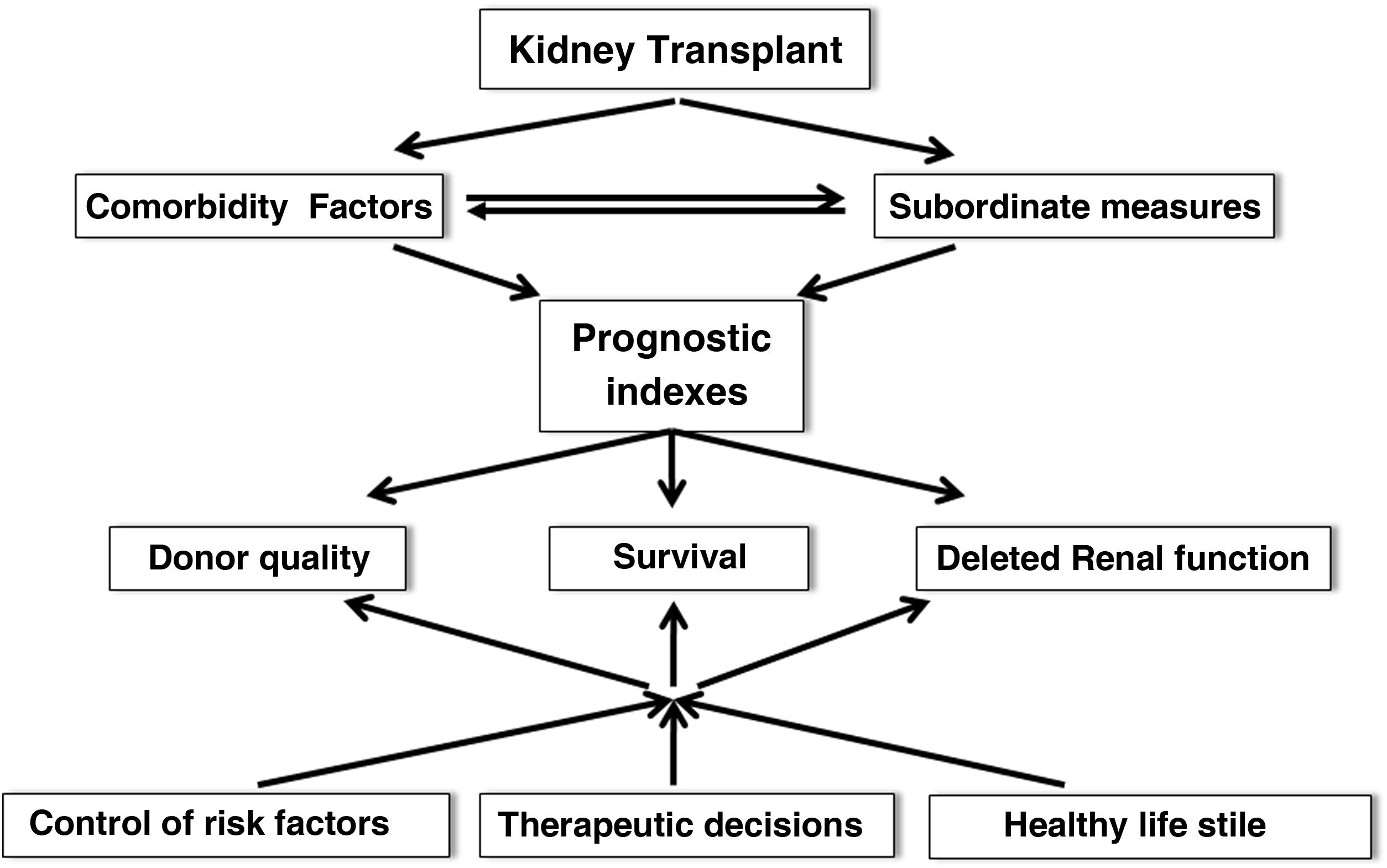
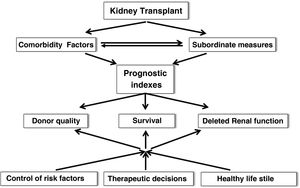
After TX there is a slow loss of graft function. Therefore, the application of prognostic indices that include comorbid risk factors and subordinate measures of survival is inexcusably needed to more accurately estimate survival, graft quality or the risk of delayed renal function (DRF) in order to make the most appropriate therapeutic decisions, recommend healthy lifestyles and individualize immunosuppression (Fig. 3). In this regard, in recent years multiple risk scores for graft loss and DRF have been generated and validated internally and externally, which could help to undertake targeted interventions to minimize the rate of graft loss after TX.19,20 In any case, it may be time to implement artificial intelligence tools (matching learning, neural networks, big data) that incorporate a myriad of data (clinical, genetic, environmental and demographic factors) to more rigorously predict graft evolution.21,22
Therapeutic strategiesSince the mid-20th century, new immunosuppressive strategies have been developed aimed at decreasing the rate of early acute rejection and minimizing TX-related complications with acceptable therapeutic yield. However, longer-term graft and patient survival have not followed a parallel course.23 Indeed, different therapeutic schemes have been tried during the last two decades to achieve such goals without detriment of patient safety (Fig. 2). Steroid withdrawal, minimization or withdrawal of CNI, double minimization of CNI and anti-mTOR drugs, new formulations of tacrolimus or the use of belatacept are some examples of these measures that aim to improve the longer-term outcomes of KT in terms of survival. In this line, early withdrawal of steroids in patients at low immunological risk optimizes the metabolic and cardiovascular profile, but this measure could facilitate the appearance of chronic graft lesions in the first two years post-TR, especially in those patients with underlying subclinical inflammation.8 Likewise, the use of reduced doses of CNI and everolimus has been shown to be very effective in preventing the rate of acute rejection and the appearance of DSA after the first two years of follow-up, with an additional lower rate of post-transplant opportunistic infections and potential cardiovascular and antitumor benefits.24 The application of projected graft survival prediction systems (iBOX system) has shown that this therapeutic regimen is not inferior to conventional therapy of a CNI plus mycophenolate mofetil in terms of survival.25 Therefore, this strategy is seen as a safe and effective therapeutic alternative for the coming years in the field of TR. Donation after cardiorespiratory arrest currently accounts for 25%–30% of all renal donations and will continue to increase in the coming years. Recipients of these grafts could benefit from receiving induction (basiliximab or thymogobulin), regardless of immunological risk. This would allow delayed administration of CNI to minimize the impact of ischemia-reperfusion and delayed graft function. Finally, parenteral use of co-stimulation blockers such as belatacept in CNI-free regimens has been shown to optimize renal function and minimize the occurrence of chronic graft dysfunction, while ensuring adherence.26 Finally, the use of extended-release tacrolimus could allow a safety profile similar to other immediate-release formulations without impairing its immunosuppressive potency.27
However, these therapeutic strategies are currently facing important clinical and socio-health challenges, such as the frequent development of DSA detected by the Luminex® technique and the increasing rate of antibody-mediated rejection, the higher incidence of long-lived donors, older patients who are sensitive to potent immunosuppressants, or the increase in health care costs, which could jeopardize the sustainability of the health care system.
What strategies are available to prolong patient survival?Prolonging patient survival is another of the great challenges for TX in the coming years, by minimizing the complications inherent to TX (cardiovascular, infectious, neoplastic and metabolic); implementing consensus documents and clinical guidelines that help in this task; optimizing the multiple predictive survival models that have been generated in this population at-risk, especially in frail patients; selecting and prioritizing patients at risk on the waiting list (WL); and applying new technologies to facilitate the clinical management of these patients.
An emerging and very frequent clinical problem that has a negative impact on the survival of patients with KT has been post-KT diabetes mellitus (PTDM). In recent years, immunosuppressive strategies have been designed to minimize this complication and the pathogenic mechanisms involved in the development of this complication have been studied in depth; these are basically based on the intracellular inhibition of the m-TOR pathway and the toxicity of the pancreatic beta cell through its potential plasticity to transform into alpha cells.28–30 Tubular sodium-glucose tubular co-transporter-2 (iSGLT2) inhibitor drugs and glucagon-like peptide-1 (GLP-1) receptor agonists have shown clear cardiovascular and renal benefit in diabetic patients with chronic kidney disease.31 Therefore, it is expected that the administration of these drugs in the coming years in diabetic patients with KT could also improve the metabolic and cardiovascular profile of this population, which will result in better survival outcomes. Likewise, early insulinization after RT could prevent the onset of PTDM, but the price to pay may be a higher rate of post-KT hypoglycemia.32 However, controlled studies in these patients are needed to provide robust scientific evidence for its clinical implementation.
Likewise, consensus documents and clinical guidelines have been drawn up by scientific societies and research networks for the management of the most frequent opportunistic infectious complications and their prevention in these patients, which could undoubtedly contribute to increasing survival in transplant patients in the next decade.33–35 The recent lessons learned from the impact generated by the COVID-19 pandemic in TX patients will also mean that in the near future we will take the appropriate therapeutic and prophylactic measures to face other upcoming pandemics with the multidisciplinary consensus of other scientific societies.
The prediction of survival through the appropriate incorporation of comorbid factors and subordinate measures in predictive models can contribute to making the best therapeutic decisions and improve survival, especially in high- and intermediate-risk patients. In the last 15 years, multiple predictive models have been developed, which predict mortality with a high concordance index, and all have been validated internally or externally in other populations.19,36 Recently, a score has been developed to predict post-TX infections through simple clinical variables that will facilitate the clinical management of these complications in the first months post-TX.37 Additionally, if these predictive models are elaborated through artificial intelligence tools, incorporating multiple data from the TX process in the different models, obviously, the predictive capacity of patient survival will be optimized, which will help to establish therapeutic schemes aimed at reducing post-TX mortality.
Despite the intense activity of KT throughout the world, WL for KT remains stable over the years.38 Mortality of WL patients is not negligible (8%–10% per year) and increases substantially in long-lived patients with higher comorbidity.39 Therefore, identifying patients at risk of death in WL could contribute to prioritizing these patients to receive a renal graft and, consequently, help to improve survival. Along these lines, in a European study conducted in 3857 patients listed for TX between 1984–2012, a composite risk model including four clinical variables (age >50 years, the presence of a central catheter at the start of dialysis and a Charlson index >3) was developed, using robust statistical models such as competing risks analysis to predict mortality in these patients, such that the risk of death increased significantly at each risk level.40 Undoubtedly, future application of artificial intelligence methods will also substantially improve predictive ability in these patients.
In addition, the detection of frailty in patients in WL for KT could also be very useful in clinical practice to identify patients at risk of early death and optimize their situation in WL through multidisciplinary prehabilitation programs developed for this purpose that include physiotherapy, nutritional measures and psychological support during the WL period. These programs could mitigate the effects of frailty and poor physical condition post-KT, and thus improve survival outcomes in these patients.41,42 Finally, the identification of WL patients at risk of developing PTDD could also be an additional measure to avoid not only comorbidity while in WL, but also during their post-TR evolution.
All this leads us to reflect on the use of new technologies in the field of KT as done in other countries in our environment, such as virtual consulting, the development of computer applications to guarantee adherence, therapeutic compliance and drug interactions, the generation of electronic devices for monitoring and control in WL, or the systematic institutionalization of advanced chronic kidney disease consultation to optimize the management of patients with very deteriorated renal function before RT. Indeed, in patients with significant loss of graft function, adequate management of immunosuppression and comorbidities associated with chronic kidney disease could decrease mortality in this population while waiting for a second chance of RT.43,44
How can we increase organ procurement and improve organ allocation?There are many kidney transplants performed in the world. Specifically, according to data from the Global Observatory on Donation and Transplantation (GOTD) in 2019, more than 100,000 TX were performed worldwide of which 37% were live donor TX, but this would only cover 10% of the current worldwide TX needs. Therefore, there is an urgent need to increase TX activity by boosting asystole donation, increasing living donor TX, promoting early TX, as well as improving organ exchange policy and optimizing clinical and histological tools for organ discard. Let us present some arguments in support of these strategies.
A recent multicenter observational study conducted in England, which reviewed a decade of experience with asystole donation, demonstrated that there was no difference in either graft survival or patient survival between encephalic death donation and donation after type III cardiac arrest after 10 years of follow-up, which opens a further door to expand the donor pool in our patients and increase TX activity.45
Living donor KT offers better results than deceased donor KT as has been shown in different observational studies.46 However, living donor KT activity has not changed substantially in recent years. On the contrary, a worrying decrease in this activity has been observed, including in our country. In view of this scenario, a working group was formed by different KT teams throughout Spain, experts in KT from the Spanish Society of Nephrology (S.E.N.), the Spanish Society of Transplantation (SET) and the ONT. This group designed a self-assessment questionnaire on living donor KT activity that was completed by the 33 living donor KT active units in Spain, with the aim of identifying potential causes of the decrease in this type of transplant activity in Spain and its possible relationship with the management of the living donor process. In this way, weaknesses, threats, strengths and opportunities (SWOT analysis) of this activity were identified, which have made it possible to draw up specific recommendations aimed at improving each of the phases of the living donation process.47 It is expected that this document could contribute to improving the activity of living donor KT in our country.
Additionally, given the mortality risk associated with remaining on dialysis, early KT can optimize survival in those patients who are expected to remain on dialysis for a long time (blood group B, young patients, hypersensitized patients, etc.). In this regard, an observational study carried out using the French organ transplant registry database with more than 22,000 patients showed that early KT offers better survival than remaining on dialysis, even for short periods of time (<6 months).48 Presumably, this could clarify many doubts about the convenience of prioritizing this activity over any time spent on dialysis.
The WL have increased in many countries in our setting and, as expected, this increases the morbidity and mortality of patients who are candidates for RT. Therefore, allocation systems could help to guarantee the usefulness of grafts and equity in access to them, which would result in a reduction in the number of patients prevalent in WL. Although dichotomous scales have been identified for correct allocation, such as the presence or absence of an expanded donor or the quantification of hypersensitized patients through the determination of panel reactive antibodies (PRA), continuous scales such as the Kidney Donor Profile Index (KDPI) or the calculation of Estimated Post-Transplant Survival (EPTS) could improve organ selection and allocation.49,50 However, these allocation systems have a moderate capacity to discriminate between similar donors, which can lead to inaccuracies in allocation. Therefore, sophisticated donor-recipient matching programs are new challenges for the future that could increase graft availability and fairness in allocation after donation.
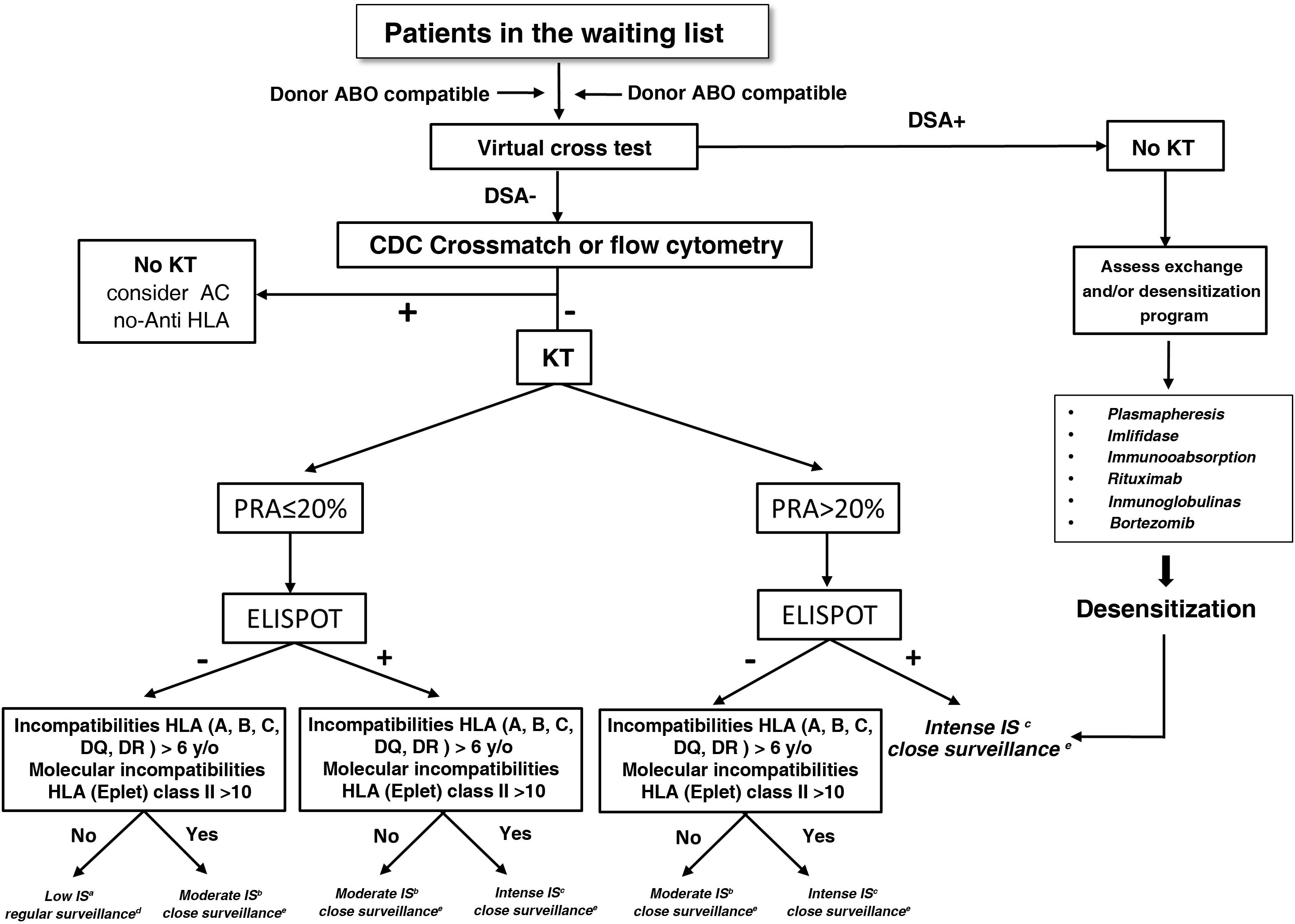
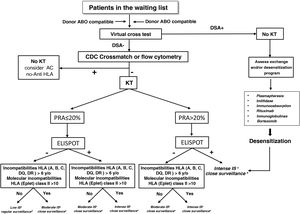
The Fig. 4 shows a proposed algorithm for organ allocation, immunosuppressive treatment and subsequent follow-up in WL patients, combining the level of humoral and cellular sensitization to human leukocyte antigens (HLA), and the degree of HLA incompatibility between donor-recipient, based on the scientific evidence indicating the different degrees of immunological risk post-KT.51–53 Humoral sensitization will be assessed using the PRA; cellular sensitization will be determined using the ELISPOT assay, and HLA incompatibility using both antigenic and molecular levels. As an example, a patient with a negative PRA plus a negative ELISPOT assay along with a low degree of incompatibility would be amenable to decreased immunosuppressive therapy. The remaining combinations of the proposed stratification would indicate the most appropriate immunosuppression regimens and post-KT follow-up according to the proposed algorithm. Likewise, in recipients with PRA>90% who do not find donors in exchange programs, in the presence of a DSA, it would also be advisable to assess pre-KT desensitizing treatment (plasmapheresis, imlifidase, immunoglobulins, etc.). Independently of the immunological risk assessment, the implementation of other algorithms based on the donor-recipient age binomial or the characteristics of donor and recipient could also facilitate organ allocation for KT and thus improve survival outcomes.
Algorithm for graft allocation, immunosuppressive scheme and post-KT monitoring according to pre-KT immunological risk.
DSA: donor-specific anti-HLA antibodies; IS: immunosuppression; PRA: panel-reactive antibodies; KT: kidney transplantation.
aLow immunosuppression includes tacrolimus plus mycophenlate mofetil or minimized doses of tacrolimus and everolimus, or tacrolimus monotherapy, without induction or maintenance prednisone.
bModerate immunosuppression: prednisone, tacrolimus and mycophenolate mofetil or mycophenolic acid with or without basiliximab induction. Alternatively prednisone and reduced doses of tacrolimus and everolimus.
cIntense immunosuppression: induction with thymoglobulin or Grafalon® plus triple therapy with prednisone, tacrolimus and mycophenolate mofetil or mycophenolic acid.
dConventional post-KT follow-up according to the characteristics of the center and the patient.
eConduct individualized monitoring of therapeutic adherence, protocol biopsies and use of specific biomarkers of immune dysfunction.
Finally, tolerance induction strategies, the development of nanomedicine, the generation of bioartificial organs through gene editing and cloning to optimize xenotransplantation, cell therapy using regulatory macrophages or the use of stem cells to obtain functioning organs, are some of the exciting future strategies to increase the organ pool and reduce the WL of our patients.54,55 However, these procedures have advantages and limitations, and many are in experimental phases far from human application. Therefore, we need multidisciplinary work and a collective effort for this to become a reality in the future decade.
Research and training of health professionals in renal transplantationResearchOur country is at the forefront of transplantation activity in the world, but clinical and basic research activity in the field of kidney transplantation does not follow parallel path, as is the case in other European countries around us. In this line, the Instituto de Salud Carlos III (ISCIII) has promoted and encouraged research in renal diseases through the creation of Research Networks, where the Renal Research Network (RedInRen) began its journey, which contemplated two major programs since its funding by the ISCIII: chronic kidney disease and renal transplantation. Specifically, the renal transplantation program was made up of 16 renal transplant groups with clinical and basic researchers distributed throughout Spain, who carry out important research activity condensed into three large blocks of work. These blocks or workpackages are coordinated by their respective leaders and bring together cutting-edge research in the clinical and basic field of KT. Personalized immunosuppression, the evaluation of the humoral response and the study of post-KT complications are at the forefront of most of the research carried out in Spain in the field of KT through the RedInRen. Currently, the ISCIII has recently reformulated the former Research Networks, transforming it into Health Outcomes-Oriented Cooperative Research Networks (RICORS) where, obviously, the renal disease research groups that have previously belonged to the RedInRen have been incorporated, including those groups with more specific lines in the field of KT.56 Apart from the individual initiatives and the Research Networks, Spanish medical research is also channeled through the Research Institutes dependent on the ISCIII, which include numerous groups belonging to them and which, through the corresponding health institutions or universities, carry out intense and prolific research activity in the field of renal diseases and KT.
Continuing education for healthcare professionalsPromoting training in transplantation is another of the great challenges for the next decade in the field of TX through training courses, workshops in methodology and statistics, spaces for the training of healthcare personnel in scientific meetings and congresses, training grants or stays in centers of excellence, but obviously to achieve this objective the support of the pharmaceutical industry, scientific societies and the healthcare administration is inexcusably needed. The Spanish Transplantation Society (SET) has been committed to this task since its origins and proof of this is the Prometeo project, which is dedicated to deepening the key issues of KT with the participation of different national groups with intense activity in KT in order to generate evidence, especially in those areas where there is a knowledge gap.
Optimize the collection of information through registersIn general, the registries of a medical or surgical activity provide essential clinical and epidemiological data to know the health status of the population and the quality and cost of medical services, guiding health managers to establish priorities and specific strategies in the health planning of different clinical entities, including the KT.57–61 Indeed, registries allow us to observe real clinical practice, provide us with essential clinical and epidemiological data (incidence, prevalence, survival), as well as quality indicators and provide information and advice to health agencies, increasing the level of evidence.62–65 They represent, therefore, a scientific and healthcare activity that contributes to improving results in any area of medicine, including KT.
There are some essential questions in the field of KT, such as survival outcomes, living donor KT activity, organ procurement policies and organ distribution strategies, or prioritization policies of WL patients for TR, that can only be adequately answered if we have accurate and truthful information collected in registries, given the long time required to obtain clinical end-points and the high consumption of resources they require. Many international and national KT registries provide very useful information on essentially epidemiological aspects such as the number of patients in WL, donor characteristics or the volume of KT performed, but not all provide detailed information on the results of KT in terms of survival or on the performance of KT programs. With this purpose, scientific and epidemiological registries have the obligation to provide information on the trajectory of KT programs and survival data, thus avoiding controversies derived from specific publications of some programs.66 With these premises, reports from KT registries can be used with guarantees and credibility by regulatory agencies, KT centers, insurance companies, patient associations and health institutions. For example, thanks to the information collected in the registries, we have been able to know the true clinical and epidemiological scope of the COVID-19 pandemic in renal patients, including WL and KT patients, throughout the world.67–71 But, undoubtedly, to achieve some of these objectives, registries must be developed through standardized systematic processes and conform be consistent with established quality standards using powerful and reliable statistical tools. In other words, they inexcusably need appropriate and powerful statistical analyses in order to generate reliable clinical data. In this sense, it is advisable to follow a checklist with the steps to follow for the proper analysis of registries and observational studies, especially when regression models are used. This can be a very useful tool for the analysis and interpretation of records.72
Furthermore, registries can increase the level of evidence by complementing wiclinical trials, which sometimes have important population-based limitations to provide strong conclusions. This can undoubtedly project registries to the highest hierarchical scale of evidence. There are now a plethora of examples of large scientific publications worldwide derived from national registries, especially the American registry, that detect health problems and risk factors for graft and KT patient survival.38,40,59,60,73
In our country we have very valuable registries, such as the ONT Donation and Transplantation Registry, the regional dialysis and KT registries, and the transplantation registries of the different scientific societies, which have been very useful to the scientific community in our country. An example of this is the valuable information provided by the ONT-SEN registry on the epidemiological and evolutionary aspects of renal patients, including patients with KT, who recently suffered the scourge of the COVID-19 pandemic.69 Undoubtedly, the integration of the different transplant registries of a country’s scientific community could increase their quality and credibility. By contrast, socio-sanitary or administrative issues that lead to the fragmentation of a country’s scientific registries could weaken their validity and reliability. The use of different or incompatible systems in the collection of information and the use of huge consumption of time and resources ratify the weakness of this strategy and do not guarantee the accuracy and completeness of the data. Therefore, it is absolutely necessary to integrate all the information in a single registry managed and guarded by the ONT, with the participation of the members of the regional registries and scientific societies, to guarantee reliable and credible information for the scientific community and the general population. In parallel, this could allow epidemiological comparisons between communities and other international registries.
ConclusionsIn the next decade we must face some challenges to further improve, if possible, the results of KT in terms of survival.
Some strategies to reduce the rate of graft loss and reduce mortality in WL and after KT could contribute to prolong the survival of these patients.
The increased activity of living donor KT, asystole donation, early transplantation and, in the not too distant future, the generation of bioartificial organs through gene editing-cloning or cell therapy could guarantee the usefulness of grafts and equity of access to them, which would allow a reduction in the number of patients on WL.
Research of excellence in the field of KT and adequate training of healthcare professionals would facilitate the implementation of strategies to improve survival outcomes.
The KT registries are very useful because they allow us to identify risk factors in this population and make it easier to compare the information with other national and inter-national registries, which can contribute to establishing strategies to improve the results of TR.
FinancingThis study was supported in part by the Spanish Ministry of Economy, Industry and Competitiveness of the Instituto de Salud Carlos III (FIS PI17/02043), co-financed by the European Regional Development Fund-FEDER, RETICS (REDINREN, RD16/0009, RD16/0009/0006).
Conflicts of interestThe authors have no conflicts of interest to declare.