As radiocephalic fistula is not necessarily appropriate for all patients with advanced kidney disease, our aim was to investigate the sensitive indicators that affect the functional primary patency of radiocephalic fistulas.
MethodsThis prospective observational study included consecutive patients referred to the Second Hospital of Dalian Medical University for initial creation of radiocephalic fistula from July 2017 to December 2019. Preoperative ultrasound parameters, demographic characteristics, serum indicators and comorbidities were recorded. The functionality of radiocephalic fistulas would be assessed every 6 months until March 2023, following their unassisted maturation, unless AVF dysfunction, kidney transplantation, mortality or loss to follow-up occurred. Kaplan–Meier analysis was employed to illustrate differences in functional primary patency of radiocephalic fistulas, while log-rank tests were utilized to compare survival curves. Univariate and multivariable Cox proportional hazard models were performed, yielding hazard ratios and 95% confidence intervals. The significance level was set at 5% for a two-sided test.
ResultsThe studies included a total of 182 patients who successfully underwent radiocephalic fistulas with primary unassisted maturation. The mean age of the study population was 58 years, with 66 percent being male. All AVFs were placed on the forearm, with 84% located on the left side.
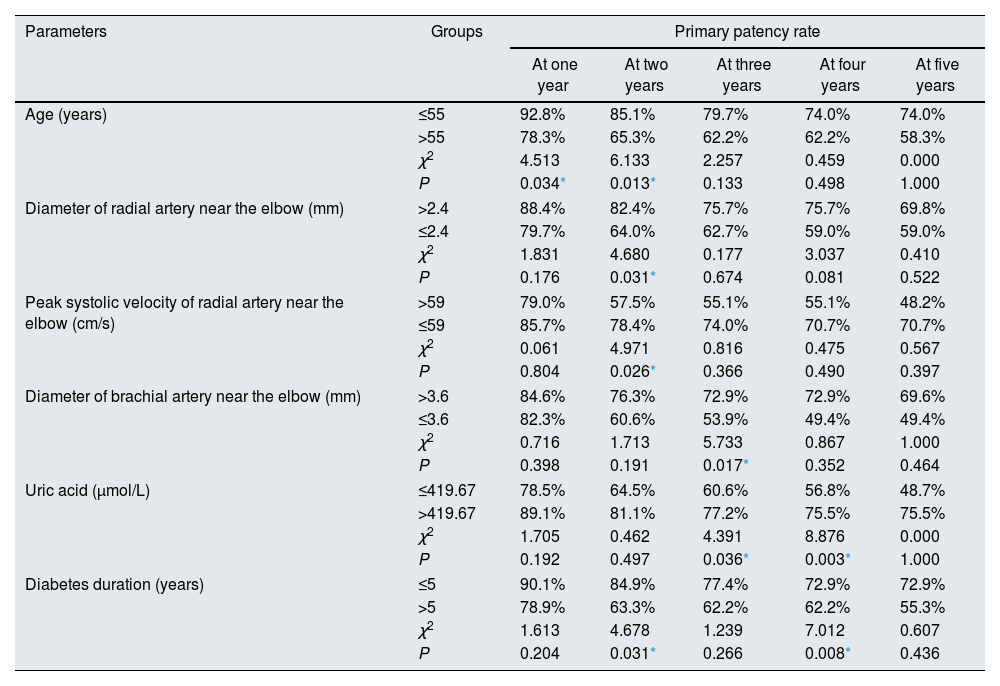
The primary patency rates of eleven parameters exhibited significant differences between groups stratified by cut-off values at different time points. Notably, the group with a peak systolic velocity of the radial artery near the elbow ≤59cm/s demonstrated a higher primary patency rate compared to the >59cm/s group at 2 years (78.4% vs 57.5%, P=0.026).
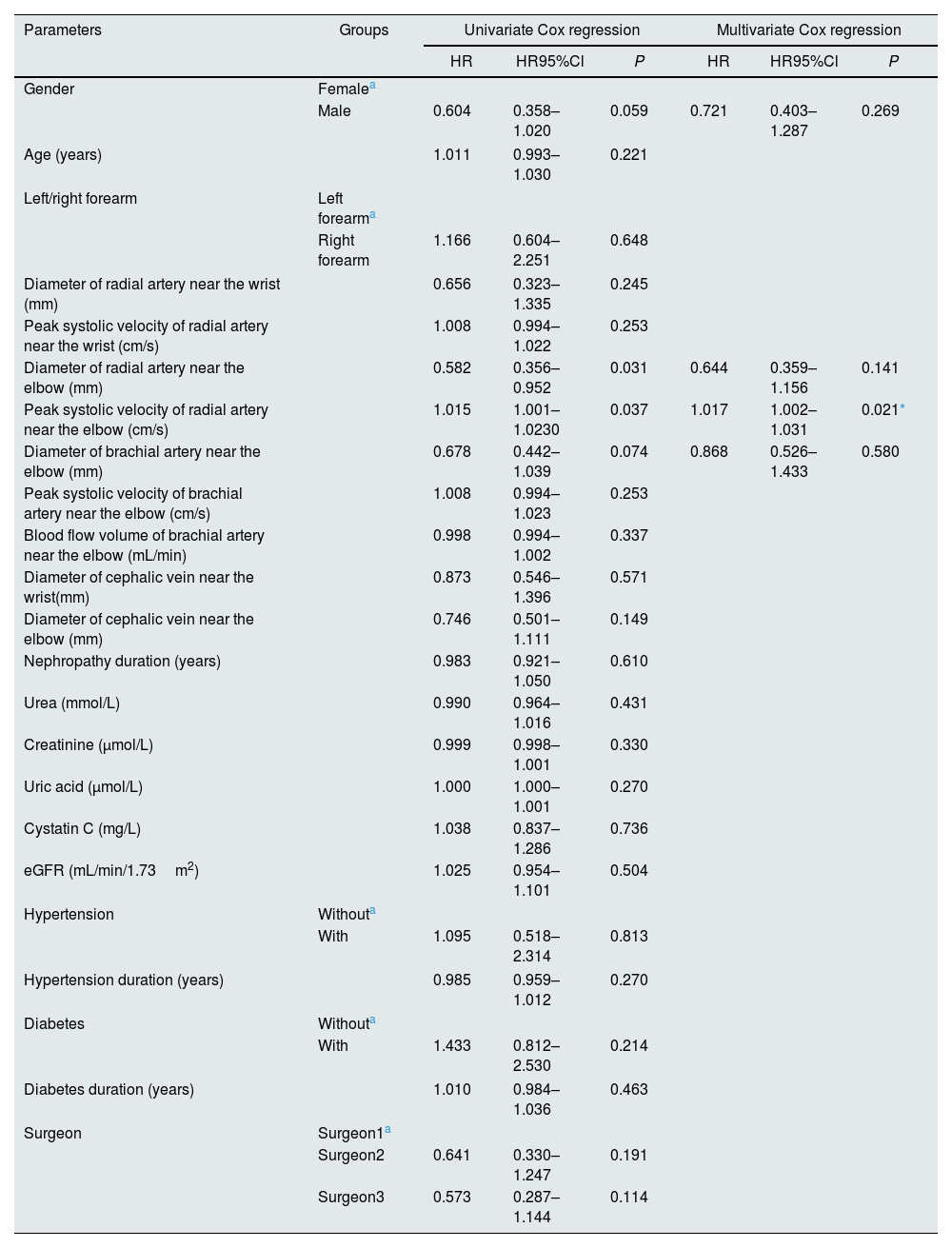
In the univariate Cox proportional hazard models, the P values for gender, the diameter of radial artery near the elbow, the peak systolic velocity of radial artery near the elbow, the diameter of brachial artery near the elbow were less than 0.1. The multivariable Cox proportional hazard model revealed that only the peak systolic velocity of radial artery near the elbow exhibited a significant impact on the functional primary patency of radiocephalic fistula (HR=1.017, 95%CI 1.002–1.031, P=0.021).
ConclusionsThe peak systolic velocity of the radial artery near the elbow is a significant risk factor for functional primary patency of radiocephalic fistula. Preoperative evaluation of the peak systolic velocity could allow to identify patients with a lower likelihood of long-term radiocephalic fistula patency, facilitating improved selection of candidates for radiocephalic fistula creation.
Dado que la fístula radiocefálica no es necesariamente adecuada para todos los pacientes con enfermedad renal avanzada, nuestro objetivo consistió en investigar los indicadores sensibles que afectan la supervivencia primaria funcional de las fístulas radiocefálicas.
MétodosEste estudio prospectivo observacional incluyó pacientes consecutivos remitidos al Segundo Hospital de la Universidad de Medicina de Dalian para someterse a la creación inicial de una fístula radiocefálica entre julio de 2017 y diciembre de 2019. Se registraron los parámetros ecográficos preoperatorios, las características demográficas, los indicadores séricos y las comorbilidades. La funcionalidad de las fístulas radiocefálicas se evaluaría cada 6 meses hasta marzo de 2023, siguiendo su maduración natural, a menos que ocurriera disfunción del acceso vascular, trasplante renal, mortalidad o pérdida en el seguimiento. Se utilizó el análisis de Kaplan-Meier para ilustrar las diferencias en la permeabilidad primaria funcional de las fístulas radiocefálicas, mientras que se emplearon pruebas log-rank para comparar las curvas de supervivencia. Se realizaron modelos univariables y multivariables mediante regresión proporcional Cox para obtener razones e intervalos de confianza del riesgo (hazard ratio) con un nivel significativo establecido en un 5% para una prueba bilateral.
ResultadosEl estudio incluyó un total de 182 pacientes que se sometieron exitosamente a fístulas radiocefálicas con maduración primaria no asistida. La edad promedio de la población estudiada fue de 58 años, con un 66 por ciento de hombres. Todas las AVF se ubicaron en el antebrazo, siendo el 84% en el lado izquierdo.
Las tasas de permeabilidad primaria para once parámetros mostraron diferencias significativas entre los grupos estratificados por valores límite en diferentes momentos. Es importante destacar que el grupo con una velocidad sistólica máxima de la arteria radial cerca del codo ≤59 cm/s demostró una tasa mayor de permeabilidad primaria en comparación con el grupo >59 cm/s a los 2 años (78,4% frente al 57,5%, p = 0,026).
En los modelos univariados de riesgo proporcional de Cox, se observaron valores de P inferiores a 0,1 para el sexo, el diámetro de la arteria radial cerca del codo, la velocidad sistólica máxima de la arteria radial cerca del codo y el diámetro de la arteria braquial cerca del codo. El modelo multivariable de riesgo proporcional de Cox reveló que únicamente la velocidad sistólica máxima de la arteria radial cerca del codo tuvo un impacto significativo en relación con la permeabilidad primaria funcional de las fístulas radiocefálicas (HR= 1,017; ic95% 1,002-1,031; P= 0,021).
ConclusionesLa velocidad sistólica máxima de la arteria radial en proximidad al codo constituye un factor de riesgo significativo para la permeabilidad primaria funcional de la fístula radiocefálica. La evaluación preoperatoria de dicha velocidad puede contribuir a identificar pacientes con una menor probabilidad de mantener una fístula radiocefálica permeable a largo plazo, lo cual facilitaría una mejor selección de candidatos para su.
As chronic kidney disease (CKD) and end-stage renal disease (ESRD) are increasing in incidence worldwide, the importance of renal replacement therapies (RRT) increases. Modes of RRT include peritoneal dialysis, renal transplant, and hemodialysis (HD),1,9,10 with hemodialysis involving autogenous arteriovenous fistula (AVF), arteriovenous grafts (AVG) and central venous catheter (CVC). Based on the 2019 KDOQI Clinical Practice Guidance Statement, selecting an appropriate RRT modality for each patient has significant implications for improving their lifetime outcomes.1
Although AVF is the preferred modality for RRT, it is far from optimal. A cautious approach is necessary to avoid indiscriminate recommendations that may result in AVF failure and subsequent interventions.2,3 Guidelines recommended various strategies to prevent AVF failure and extend its lifespan, including appropriate pre-operative patient selection.3 We intend to evaluate whether AVF is the appropriate modality for the patient and whether the patient is suitable for AVF before surgery. Due to the high incidence of primary maturation failure in AVFs, previous studies have predominantly focused on investigating the AVF maturation, with relatively limited research conducted on patency, most of which has centered around postoperative parameters.4–8 One study examined the impact of postoperative measurements on primary patency and found that AVF diameter was the only predictor of patency.8 Another study on the prediction of AVF dialysis use by ultrasound examination after fistula creation concluded that a diameter above 5mm and an arterial end-diastolic velocity above 110cm/s were the best predictors of dialysis use.4 To our knowledge, there is a scarcity of research exploring how preoperative parameters influence the outcome of AVF.
Additionally, different literatures employed various terminologies to describe outcomes of AVF1,9 and three failure outcomes of AVF were considered: early thrombosis, failure to mature, and late failure.3 This study chose to assess the functional primary patency of RCAVFs following unassisted maturation without early thrombosis, with a particular focus on late failure. And it should be noted that AVF encompasses different types such as radiocephalic fistula (RCAVF), brachiocephalic fistula (BCAVF) and brachiobasilic fistula (BBAVF), etc., and some previous studies did not specify a particular type.4,5,8 Considering that RCAVF is the preferred method with the highest case volume in our hospital, this study will primarily focus on investigating it.
In conclusion, we aim to identify sensitive indicators capable of predicting the functional primary patency of RCAVFs, with the intention of enhancing preoperative modality selection and patient screening. Patients with a lower likehood of long-term functional patency could avoid unnecessary surgery and benefit from alternative methods of RRT.
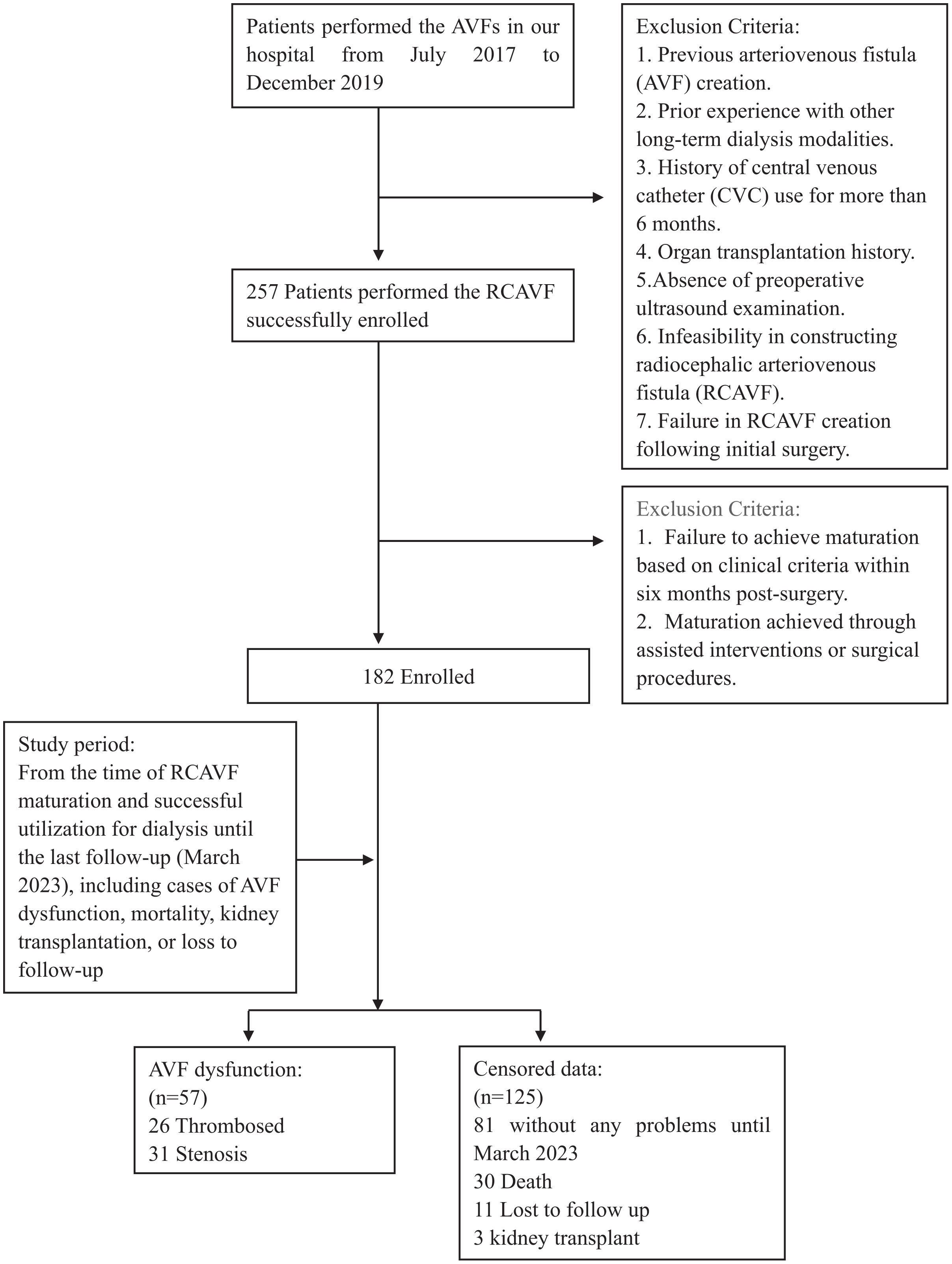
Materials and methodsStudy populationThis prospective observational study was conducted from July 2017 to March 2023. Initially, all patients scheduled for primary RCAVF creation at the Second Hospital of Dalian Medical University were enrolled between July 2017 and December 2019. The exclusion criteria encompassed patients with permanent access or prior exposure to other dialysis modalities like peritoneal dialysis, and those who had undergone central vein catheter (CVC) for more than six months. Patients who did not develop a surgical anastomosis between the radial artery and the cephalic vein or those whose initial creation was unsuccessful were excluded from the study. Once RCAVFs reached maturation and were successfully used for dialysis, patients would be followed up every six months until March 2023, unless RCAVF dysfunction occurred, death ensued, or individuals were lost to follow-up. The follow-up duration varied based on patients’ creation date, to ensure accurate outcomes, patients without any endpoints during the study were followed for at least 39 months. The remaining patients with immature RCAVFs were excluded. The flowchart (Fig. 1) provides the detailed information. The University Hospital Ethics Committee granted an exemption for ethical approval, and informed consent was obtained from all participants.
Clinical data collectionThe clinical study involved the collection and recording of clinical data, encompassing patient demographics, comorbidities such as hypertension, diabetes mellitus, and kidney disease along with their progression, as well as preoperative serum indicators (serum creatinine, blood urea, uric acid, serum cystatin C and estimated glomerular filtration rate (eGFR). All serum indicators were obtained within one week before RCAVF creation.
Ultrasound examinationsAll patients enrolled in the study underwent routine vascular ultrasound examination within one week before RCAVF creation. The ultrasound examinations were performed by a single vascular sonographer with approximately 8 years of experience in US on AVF, using an Aplio-500 (Toshiba Medical System Corporation, Tokyo, Japan) device and a linear vascular probe 5–14-MHz probe. During the procedure, the patient assumed a supine position with the investigated arm extended alongside their body and palm facing upwards at room temperature. A tourniquet was applied to the upper arm and patients were instructed to clench their fists. The internal diameter of the cephalic vein and radial artery was measured at two locations, namely near the wrist (distal forearm, 3cm from the wrist crease) and near the elbow (proximal forearm, 1cm to the cubital fossa), in a transverse section. The measurements were obtained from the interface between vessel wall and lumen, extending from near to far wall. Additionally, spectral Doppler was used to evaluate the radial artery longitudinally, capturing PSV at two locations where the diameter was measured. The Doppler range gate encompassed the entire lumen with an insonation angle set at 60 degrees or less. The diameter and PSV of the brachial artery were measured at the cubital fossa using the same method used for the radial artery. After analyzing at least three cardiac cycles by the ultrasound system, blood flow of brachial artery was automatically calculated from the Doppler trace. Following the vessel measurements, pre-operative vessel mapping was performed by the same operator.
Creation and maturation of RCAVFThe creation of RCAVF was performed by one of three experienced nephrologists, each with a minimum of 10 years’ experience in RCAVF creation. The vascular access plan was determined based on physical examination, vessel mapping and ultrasound measurements. Whenever both right and left forearms were suitable for creation, attempts were made to create RCAVFs on the nondominant hand. The surgical procedure was performed on all patients under local anesthesia. The incisions were routinely made 3cm above the wrist. An end-to-side arteriovenous anastomosis was created between the radial artery and cephalic vein. Successful fistula creation is confirmed by physical examination. At 4–8 weeks after creation, all patients underwent screening by a nephrologist at our dialysis unit, if no thrill was detected in the RCAVFs, patients were referred for a more detailed ultrasound evaluation. The maturation of RCAVF was assessed based on clinical criteria, which encompassed providing blood flow exceeding 200mL/min for a minimum of three consecutive weekly hemodialysis sessions, enabling convenient needle insertion during dialysis while minimizing bleeding risk. The patients who achieved unassisted RCAVF maturation and consistently used it for dialysis within six months were eligible for inclusion. The fistulas were assessed every time before dialysis by the nursing staff to identify any clinical indications of dysfunction, and a routine feedback was provided to us every six months. In case the patients opted to transfer to another dialysis unit post-maturation, they would be subjected to telephone and WeChat follow-ups every six months. The functional primary patency of RCAVF was determined as the duration between successful and regular use of a mature RCAVF for dialysis and the confirmation of first instance of access dysfunction. Patients who maintained RCAVF functionality were followed until the study's end, unless they died, underwent kidney transplantation, or were lost to follow-up.
Statistical analysisStatistical analyses were performed using SPSS, version 23.0 (SPSS, Chicago, IL, USA) and MedCalc for Windows, version 19.0 (MedCalc Software, Mariakerke, Belgium). The continuous variables are presented as mean (standard deviation), or median (interquartile range) in cases where the values did not exhibit a normal distribution. Categorical variables are presented as percentages. Nonparametric tests (Wilcoxon Mann–Whitney or Kruskal–Wallis) were used for continuous variables while the Chi-square test was applied to assess the categorical data. Continuous variables were categorized based on predictive cut-off points determined by generating receiver operating characteristic (ROC) curves. Kaplan–Meier curves were used to illustrate survival differences between groups and log-rank tests were performed to compare survival curves.
The association between the parameters and functional primary patency was initially explored using univariate Cox proportional hazard models, followed by multivariable models to identify the independent predictors of fistula functional primary patency, including only those with a P-value<0.1 in the univariate analyses. Hazard ratios (HRs) along with their corresponding 95% confidence intervals (CIs) were used to estimate risks in these models. Statistical significance was defined as P<0.05.
ResultsA total of 257 patients with ESRD underwent successful placement of RCAVF from July 2017 to December 2019, with pre-operative ultrasound. Follow up for 6 months, 75 (29.2%) failed to mature for the first time. The final cohort included the remaining 182 patients (70.8%) with a RCAVF that matured without any assisted invention or surgery. The interval between the access placement and RCAVF maturation ranged from 1 to 6 months (mean 2.4 months). Among the 182 patients, failure was observed in 57 cases during the follow-up period, primarily attributed to thrombosis (n=2614.3%) or stenosis (n=31.17.0%). The functional primary patency of RCAVFs last on average 30.9 months (range, 2–66). At the end of study, 81 remained on RCAVFs, 30 had died, 11 had been lost to follow-up and 3 had received a kidney transplant.
The study population comprised mostly of males (65.9%) with a mean age of 58 years (range, 24–90). All AVFs were placed in the forearm, with 84% placed on the left side. Among the patients, 116 (64%) had diabetes and 155 (85%) had hypertension for an average duration of 9.8 and 9.9 years respectively. The demographic data is presented in Tables 1 and 2.
Baseline characteristics of the quantitative variables of the patients before the placement of RCAVFs.
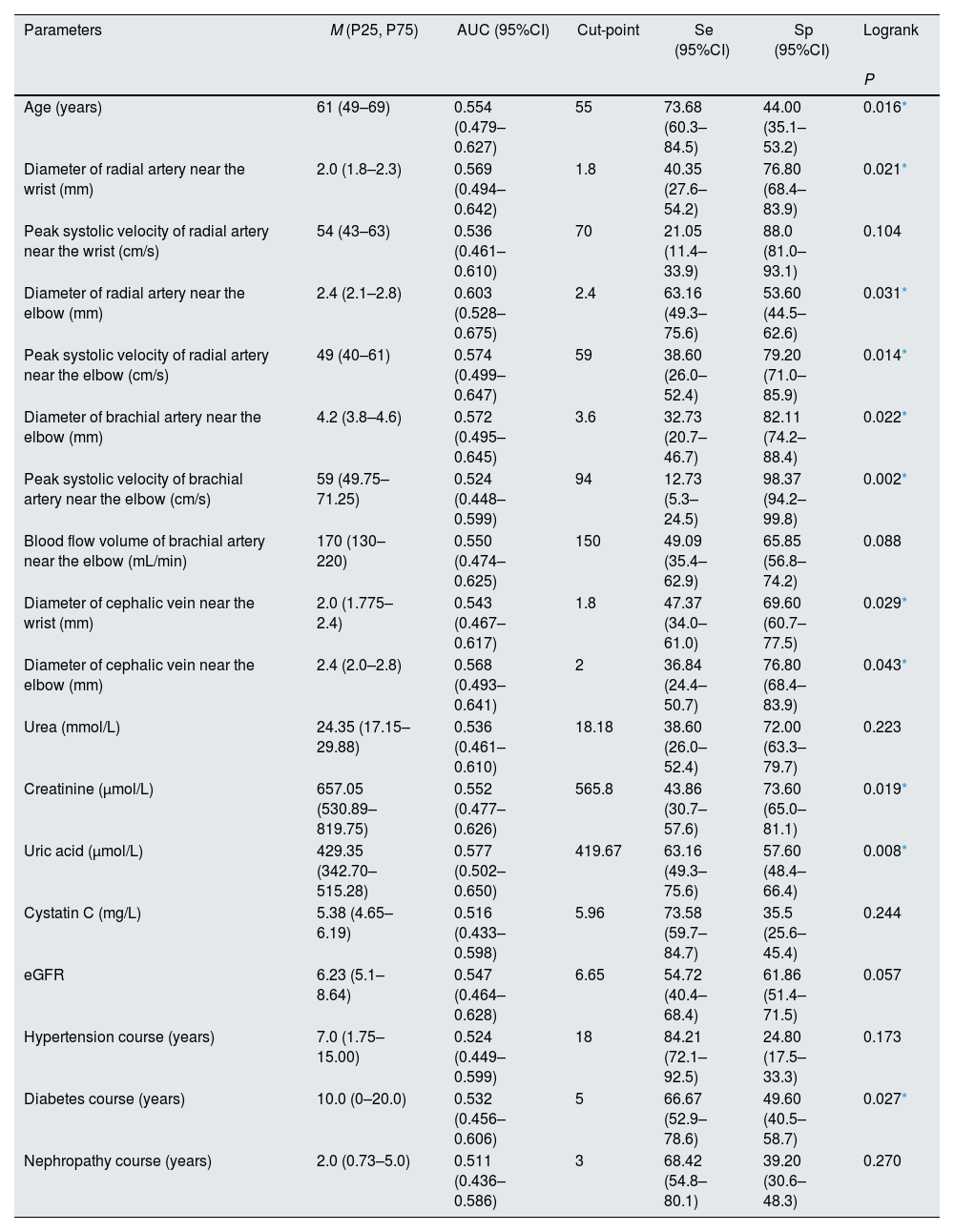
| Parameters | M (P25, P75) | AUC (95%CI) | Cut-point | Se (95%CI) | Sp (95%CI) | Logrank |
|---|---|---|---|---|---|---|
| P | ||||||
| Age (years) | 61 (49–69) | 0.554 (0.479–0.627) | 55 | 73.68 (60.3–84.5) | 44.00 (35.1–53.2) | 0.016* |
| Diameter of radial artery near the wrist (mm) | 2.0 (1.8–2.3) | 0.569 (0.494–0.642) | 1.8 | 40.35 (27.6–54.2) | 76.80 (68.4–83.9) | 0.021* |
| Peak systolic velocity of radial artery near the wrist (cm/s) | 54 (43–63) | 0.536 (0.461–0.610) | 70 | 21.05 (11.4–33.9) | 88.0 (81.0–93.1) | 0.104 |
| Diameter of radial artery near the elbow (mm) | 2.4 (2.1–2.8) | 0.603 (0.528–0.675) | 2.4 | 63.16 (49.3–75.6) | 53.60 (44.5–62.6) | 0.031* |
| Peak systolic velocity of radial artery near the elbow (cm/s) | 49 (40–61) | 0.574 (0.499–0.647) | 59 | 38.60 (26.0–52.4) | 79.20 (71.0–85.9) | 0.014* |
| Diameter of brachial artery near the elbow (mm) | 4.2 (3.8–4.6) | 0.572 (0.495–0.645) | 3.6 | 32.73 (20.7–46.7) | 82.11 (74.2–88.4) | 0.022* |
| Peak systolic velocity of brachial artery near the elbow (cm/s) | 59 (49.75–71.25) | 0.524 (0.448–0.599) | 94 | 12.73 (5.3–24.5) | 98.37 (94.2–99.8) | 0.002* |
| Blood flow volume of brachial artery near the elbow (mL/min) | 170 (130–220) | 0.550 (0.474–0.625) | 150 | 49.09 (35.4–62.9) | 65.85 (56.8–74.2) | 0.088 |
| Diameter of cephalic vein near the wrist (mm) | 2.0 (1.775–2.4) | 0.543 (0.467–0.617) | 1.8 | 47.37 (34.0–61.0) | 69.60 (60.7–77.5) | 0.029* |
| Diameter of cephalic vein near the elbow (mm) | 2.4 (2.0–2.8) | 0.568 (0.493–0.641) | 2 | 36.84 (24.4–50.7) | 76.80 (68.4–83.9) | 0.043* |
| Urea (mmol/L) | 24.35 (17.15–29.88) | 0.536 (0.461–0.610) | 18.18 | 38.60 (26.0–52.4) | 72.00 (63.3–79.7) | 0.223 |
| Creatinine (μmol/L) | 657.05 (530.89–819.75) | 0.552 (0.477–0.626) | 565.8 | 43.86 (30.7–57.6) | 73.60 (65.0–81.1) | 0.019* |
| Uric acid (μmol/L) | 429.35 (342.70–515.28) | 0.577 (0.502–0.650) | 419.67 | 63.16 (49.3–75.6) | 57.60 (48.4–66.4) | 0.008* |
| Cystatin C (mg/L) | 5.38 (4.65–6.19) | 0.516 (0.433–0.598) | 5.96 | 73.58 (59.7–84.7) | 35.5 (25.6–45.4) | 0.244 |
| eGFR | 6.23 (5.1–8.64) | 0.547 (0.464–0.628) | 6.65 | 54.72 (40.4–68.4) | 61.86 (51.4–71.5) | 0.057 |
| Hypertension course (years) | 7.0 (1.75–15.00) | 0.524 (0.449–0.599) | 18 | 84.21 (72.1–92.5) | 24.80 (17.5–33.3) | 0.173 |
| Diabetes course (years) | 10.0 (0–20.0) | 0.532 (0.456–0.606) | 5 | 66.67 (52.9–78.6) | 49.60 (40.5–58.7) | 0.027* |
| Nephropathy course (years) | 2.0 (0.73–5.0) | 0.511 (0.436–0.586) | 3 | 68.42 (54.8–80.1) | 39.20 (30.6–48.3) | 0.270 |
Baseline characteristics of the qualitative variables of the patients before the placement of RCAVFs.
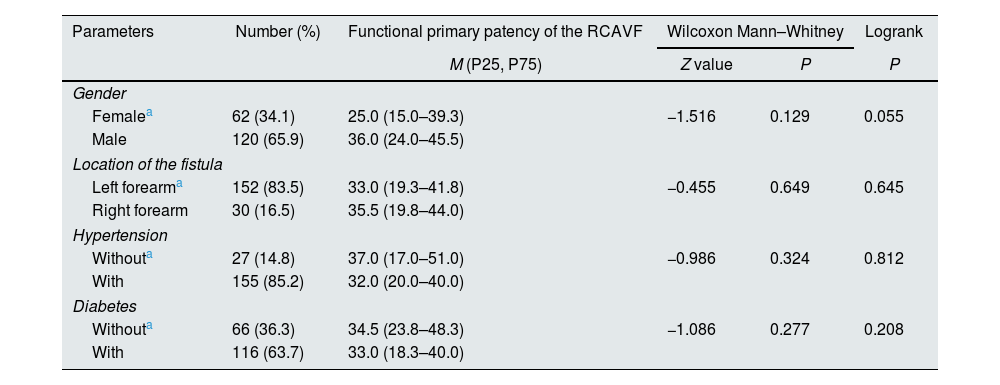
| Parameters | Number (%) | Functional primary patency of the RCAVF | Wilcoxon Mann–Whitney | Logrank | |
|---|---|---|---|---|---|
| M (P25, P75) | Z value | P | P | ||
| Gender | |||||
| Femalea | 62 (34.1) | 25.0 (15.0–39.3) | −1.516 | 0.129 | 0.055 |
| Male | 120 (65.9) | 36.0 (24.0–45.5) | |||
| Location of the fistula | |||||
| Left forearma | 152 (83.5) | 33.0 (19.3–41.8) | −0.455 | 0.649 | 0.645 |
| Right forearm | 30 (16.5) | 35.5 (19.8–44.0) | |||
| Hypertension | |||||
| Withouta | 27 (14.8) | 37.0 (17.0–51.0) | −0.986 | 0.324 | 0.812 |
| With | 155 (85.2) | 32.0 (20.0–40.0) | |||
| Diabetes | |||||
| Withouta | 66 (36.3) | 34.5 (23.8–48.3) | −1.086 | 0.277 | 0.208 |
| With | 116 (63.7) | 33.0 (18.3–40.0) | |||
| Kruskal–Wallis | Logrank | ||||
|---|---|---|---|---|---|
| H value | P | P | |||
| Surgeon | |||||
| Surgeon 1a | 77 (42.0) | 31.0 (24.0–44.5) | 0.606 | 0.739 | 0.247 |
| Surgeon 2 | 73 (40.3) | 37.0 (18.5–41.0) | |||
| Surgeon 3 | 32 (17.7) | 24.5 (13.5–46.3) | |||
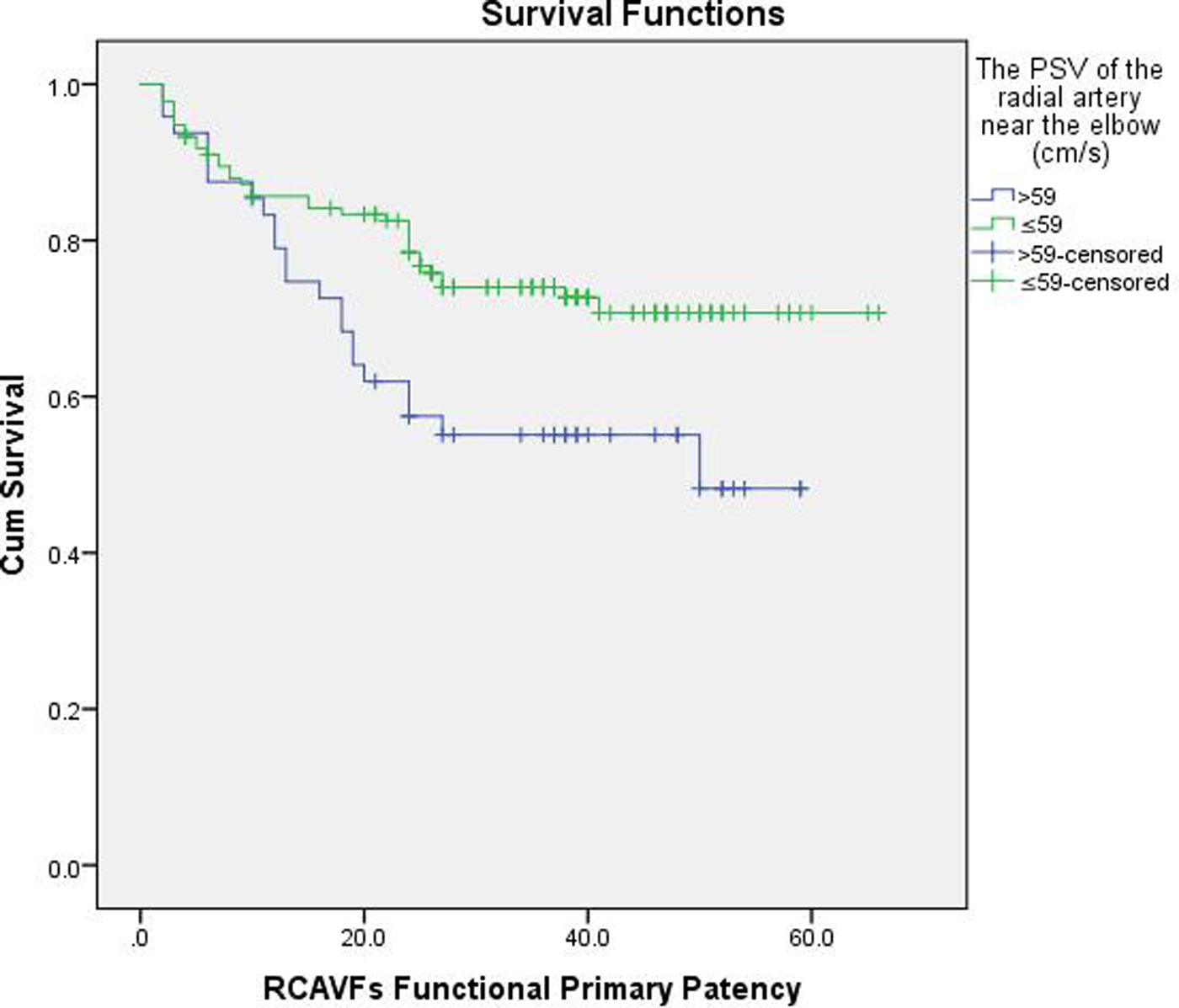
According to the cut-points determined by ROC analyses, the parameters were divided into 2 groups: > cut-point and ≦ cut-point groups. Eleven parameters show significant differences between groups, as summarized in Table 1. Among the aforementioned eleven parameters, there were significant differences observed in the functional primary patency rates of six parameters across different years. At 2 years, the group with a PSV of radial artery near the elbow ≤59cm/s exhibited a higher primary patency rate compared to >59cm/s group (78.4% vs 57.5%, P=0.026) (Fig. 2). In the >59cm/s group, there was a significantly higher probability of thrombosis or stenosis formation (45.8% patients, 22 out of 48) compared to the ≤59cm/s group (26.1%, 35 out of 134), with a statistically significant difference observed (P=0.012). Additional relevant data can be found in Table 3.
Primary patency rate of parameters exhibiting significant intergroup differences.
| Parameters | Groups | Primary patency rate | ||||
|---|---|---|---|---|---|---|
| At one year | At two years | At three years | At four years | At five years | ||
| Age (years) | ≤55 | 92.8% | 85.1% | 79.7% | 74.0% | 74.0% |
| >55 | 78.3% | 65.3% | 62.2% | 62.2% | 58.3% | |
| χ2 | 4.513 | 6.133 | 2.257 | 0.459 | 0.000 | |
| P | 0.034* | 0.013* | 0.133 | 0.498 | 1.000 | |
| Diameter of radial artery near the elbow (mm) | >2.4 | 88.4% | 82.4% | 75.7% | 75.7% | 69.8% |
| ≤2.4 | 79.7% | 64.0% | 62.7% | 59.0% | 59.0% | |
| χ2 | 1.831 | 4.680 | 0.177 | 3.037 | 0.410 | |
| P | 0.176 | 0.031* | 0.674 | 0.081 | 0.522 | |
| Peak systolic velocity of radial artery near the elbow (cm/s) | >59 | 79.0% | 57.5% | 55.1% | 55.1% | 48.2% |
| ≤59 | 85.7% | 78.4% | 74.0% | 70.7% | 70.7% | |
| χ2 | 0.061 | 4.971 | 0.816 | 0.475 | 0.567 | |
| P | 0.804 | 0.026* | 0.366 | 0.490 | 0.397 | |
| Diameter of brachial artery near the elbow (mm) | >3.6 | 84.6% | 76.3% | 72.9% | 72.9% | 69.6% |
| ≤3.6 | 82.3% | 60.6% | 53.9% | 49.4% | 49.4% | |
| χ2 | 0.716 | 1.713 | 5.733 | 0.867 | 1.000 | |
| P | 0.398 | 0.191 | 0.017* | 0.352 | 0.464 | |
| Uric acid (μmol/L) | ≤419.67 | 78.5% | 64.5% | 60.6% | 56.8% | 48.7% |
| >419.67 | 89.1% | 81.1% | 77.2% | 75.5% | 75.5% | |
| χ2 | 1.705 | 0.462 | 4.391 | 8.876 | 0.000 | |
| P | 0.192 | 0.497 | 0.036* | 0.003* | 1.000 | |
| Diabetes duration (years) | ≤5 | 90.1% | 84.9% | 77.4% | 72.9% | 72.9% |
| >5 | 78.9% | 63.3% | 62.2% | 62.2% | 55.3% | |
| χ2 | 1.613 | 4.678 | 1.239 | 7.012 | 0.607 | |
| P | 0.204 | 0.031* | 0.266 | 0.008* | 0.436 | |
The univariate Cox proportional hazard models showed the parameters with a P-value of less than 0.1 were gender, the diameter of radial artery near the elbow, the PSV of radial artery near the elbow, and the diameter of brachial artery near the elbow (gender: HR=0.604, 95%CI 0.358–1.020, P=0.059, the diameter of radial artery near the elbow: HR=0.582, 95%CI 0.356–0.952, P=0.031, the PSV of radial artery near the elbow: HR=1.015, 95%CI 1.001–1.030, P=0.037, the diameter of brachial artery near the elbow: HR=0.678, 95%CI 0.442–1.039, P=0.074). All four parameters were included in the multivariable Cox regression analysis, and only the effect of the PSV of radial artery near the elbow had a statistically significant effect on RCAVF functional primary patency (HR=1.017, 95%CI 1.002–1.031, P=0.021). The other three parameters did not show statistical significance (P>0.05). The details can be found in Table 4.
Postmatuarion RCAVF primary patency for patients: Univariate and Multivariate Cox regression analysis.
| Parameters | Groups | Univariate Cox regression | Multivariate Cox regression | ||||
|---|---|---|---|---|---|---|---|
| HR | HR95%Cl | P | HR | HR95%Cl | P | ||
| Gender | Femalea | ||||||
| Male | 0.604 | 0.358–1.020 | 0.059 | 0.721 | 0.403–1.287 | 0.269 | |
| Age (years) | 1.011 | 0.993–1.030 | 0.221 | ||||
| Left/right forearm | Left forearma | ||||||
| Right forearm | 1.166 | 0.604–2.251 | 0.648 | ||||
| Diameter of radial artery near the wrist (mm) | 0.656 | 0.323–1.335 | 0.245 | ||||
| Peak systolic velocity of radial artery near the wrist (cm/s) | 1.008 | 0.994–1.022 | 0.253 | ||||
| Diameter of radial artery near the elbow (mm) | 0.582 | 0.356–0.952 | 0.031 | 0.644 | 0.359–1.156 | 0.141 | |
| Peak systolic velocity of radial artery near the elbow (cm/s) | 1.015 | 1.001–1.0230 | 0.037 | 1.017 | 1.002–1.031 | 0.021* | |
| Diameter of brachial artery near the elbow (mm) | 0.678 | 0.442–1.039 | 0.074 | 0.868 | 0.526–1.433 | 0.580 | |
| Peak systolic velocity of brachial artery near the elbow (cm/s) | 1.008 | 0.994–1.023 | 0.253 | ||||
| Blood flow volume of brachial artery near the elbow (mL/min) | 0.998 | 0.994–1.002 | 0.337 | ||||
| Diameter of cephalic vein near the wrist(mm) | 0.873 | 0.546–1.396 | 0.571 | ||||
| Diameter of cephalic vein near the elbow (mm) | 0.746 | 0.501–1.111 | 0.149 | ||||
| Nephropathy duration (years) | 0.983 | 0.921–1.050 | 0.610 | ||||
| Urea (mmol/L) | 0.990 | 0.964–1.016 | 0.431 | ||||
| Creatinine (μmol/L) | 0.999 | 0.998–1.001 | 0.330 | ||||
| Uric acid (μmol/L) | 1.000 | 1.000–1.001 | 0.270 | ||||
| Cystatin C (mg/L) | 1.038 | 0.837–1.286 | 0.736 | ||||
| eGFR (mL/min/1.73m2) | 1.025 | 0.954–1.101 | 0.504 | ||||
| Hypertension | Withouta | ||||||
| With | 1.095 | 0.518–2.314 | 0.813 | ||||
| Hypertension duration (years) | 0.985 | 0.959–1.012 | 0.270 | ||||
| Diabetes | Withouta | ||||||
| With | 1.433 | 0.812–2.530 | 0.214 | ||||
| Diabetes duration (years) | 1.010 | 0.984–1.036 | 0.463 | ||||
| Surgeon | Surgeon1a | ||||||
| Surgeon2 | 0.641 | 0.330–1.247 | 0.191 | ||||
| Surgeon3 | 0.573 | 0.287–1.144 | 0.114 | ||||
Based on the findings of this study, the PSV of the radial artery near the elbow can be considered an independent risk factor of functional primary patency for RCAVFs. The hazard ratio is greater than 1, indicating a 1.7% increased risk of primary late-failure in RCAVF per unit increase in PSV. Using a cut-off value of 59cm/s for PSV in the radial artery near the elbow, evidence suggests that patients with ≤59cm/s have better overall primary patency rates compared to those with >59cm/s, particularly at 2 years where a statistically significant difference was observed. PSV of radial arteries near the elbow above 59cm/s could predict relatively shorter functional primary patency of RCAVF.
The Spanish Clinical Guidelines for Vascular Access in Hemodialysis10 propose that peak systolic velocity, as a flow characteristic at the arterial level, exhibits a certain degree of correlation in predicting future arteriovenous fistula (AVF) development. However, there is limited existing research on the assessment of peak systolic velocity of arteries. According to the literature available, only one earlier study briefly mentioned a contrary finding proposing that a PSV of radial artery at least 50cm/s could serve as a criterion for AVF placement.11 This criterion was also briefly mentioned in two reviews published in 201612 and 2022.13 The likely reason is that the minimum standard for PSV aims to optimize blood flow and reduce the likelihood of blood clots, which exerts a more pronounced influence on AVF creation and early thrombosis prevention.14 However, our study specifically focused on the post-maturation patency, which may account for the divergent findings.
A previous study proposed that systolic spikes and high pulsatility index (PI) were identified as risk factors for AVF failure, with a higher probability of failure observed in AVFs with PI greater than 1.4.15 It's probably because that an elevated systolic spikes may contribute to a more rapid increase and fluctuation in wall shear stress (WSS), which refers to the traction stress induced by velocity gradient on the luminal vessel surface.16 Andrea et al.16 suggested that unsteady and disturbed WSS patterns could stimulate endothelial cells (ECs), leading to intimal hyperplasia development and induction of a proinflammatory state, thereby promoting inward remodeling instead of outward remodeling of vessels, ultimately impeding AVF maturation and resulting in primary failure. Perhaps this is the underlying reason why the higher PSV of the radial artery hinders the suitability of utilizing RCAVF.
Notably, the measurement location in the previous studies was typically set near the wrist. A previous research had identified that the diameter of radial artery and cephalic vein (measured at about 3–6cm above the wrist) were risk factors for surgical failure.15 Another research concluded that the diameter of the artery was the sole critical factor associated with the maturation of AVF, in which the measurement point was established at a distance of 5cm above the wrist.17 The diameter near the wrist may have a more significant impact on surgical procedures and maturation, but it may not be associated with subsequent patency. As observed in our study, there was no correlation between the diameter near the wrist and functional primary patency in either multivariable or univariate analysis. Similarly, when compared to assessing PSV near the wrist, evaluating PSV near the elbow has potential for providing a more comprehensive evaluation of subsequent arterial supply and expansion. Regarding the radial artery diameter near the elbow, it only showed a significant correlation (P-value<0.05) in univariate Cox regression analysis, but no correlation was found in multivariable analysis. This discrepancy could be attributed to an indirect association formed by PSV with patency or possibly due to limited sample size. Unfortunately, there have been limited studies that have separately investigated the two positions, near the wrist and near the elbow. This dearth of research may partially explain why previous studies did not identify a significant correlation with PSV.
In addition to ultrasound parameters, this study comprehensively incorporates preoperative factors that may be relevant to AVF, including routine indicators such as patient demographics and comorbidities, as well as less commonly included factors like serum markers, aiming to provide a comprehensive assessment. Previous studies have suggested that serum markers like eGFR hold potential in reflecting the optimal timing for AVF creation.1,18 Additionally, there is literature indicating that Uremia significantly exacerbates inflammation and oxidative stress, both of which impact the formation of NIH and endothelial function, ultimately leading to a prothrombotic state.3 Furthermore, another study has also supported the influence of the uremic state in patients with ESRD on AVF outcomes, with higher levels of serum urea being associated with poorer patency at 6 weeks and inferior long-term outcomes in RCAVF.19 However, our study did not find a significant correlation between serum indicators and the functional primary patency of RCAVFs. Consistent with our findings, previous studies have also failed to establish associations between preoperative blood urea nitrogen, eGFR, and serum creatinine levels with maturation and functionality.17,20 A single or multiple serum indicators may not offer a comprehensive reflection of the overall patient condition and could be influenced by temporary dialysis before surgery.17
Conversely, arterial PSV might provide insights into the long-term preoperative state of patients. The presence of systemic abnormalities in ESRD patients has been reported to contribute to the development of accelerated atherosclerosis, vessel thickening, vascular calcification, and stiffness,3,21 which might lead to PSV of the artery elevated. A higher PSV likely indicates more severe pre-existing vascular pathology, which is closely associated with AVF maturation and patency.22 This perspective also could explain why a higher PSV negatively impacts the patency of RCAVF.
Naturally, as indicated in the Spanish Clinical Guidelines,10 it is not advisable to base vascular access decisions solely on one clinical or ultrasound parameter. It is recommended that a comprehensive evaluation of the patient's medical history, vascular physical examination, and preoperative ultrasound be conducted, taking into account individual preferences. Therefore, the decision to undergo arteriovenous fistula surgery should not rely solely on PSV of radial artery, but rather consider other factors in conjunction. A thorough assessment of various aspects of each patient's condition should be undertaken to tailor an optimal dialysis plan for them. The significance of this study's findings lies in alerting nephrologists to the potential detrimental effects excessive PSV may have on functional primary patency, as previous researches have yielded limited relevant results and primarily focused on studying diameter thresholds.
There are several limitations to our study as well. Firstly, 38 (20.9%) patients opted for transferring to another dialysis unit after maturation, their outcomes were assessed via telephone and WeChat. It should be noted that the inclusion of these patients may have potentially compromised the accuracy of the results. Secondly, the objective of this study is to evaluate the suitability of patients for creating RCAVF preoperatively, so we have exclusively included preoperative parameters while not addressing the impact of postoperative parameters on functional primary patency. However, it should be noted that postoperative parameters such as ultrasound results after surgery, puncture techniques, dialysis guidelines, prescribed flows of the pump, among others can all potentially influence the functional primary patency of RCAVFs. Therefore, we intend to incorporate these postoperative parameters in future studies. Additionally, since this study only focuses on RCAVF, it is possible that patients with more severe conditions and complications, who were unsuitable for creating RCAVF, have already been excluded. This may potentially affect the generalizability of our findings. In the future studies, we would include other different modalities of AVF to validate our results. Lastly, an increase in PSV can be expected due to decreased arterial compliance and pre-existing vascular disease associated with common risk factors such as advanced age, gender, hypertension, diabetes and presence of uremia. Although this study incorporates these variables in the analysis, it remains challenging to completely eliminate their impact on PSV of the arteries. However, PSV may serve as a more comprehensive indicator of patients’ overall physical condition, as previously discussed.
ConclusionThe aim of this study was to assess patients’ suitability for RCAVF by comprehensively investigating the impact of pre-operative parameters on the functional primary patency of unassisted matured RCAVF. This study concluded that the PSV of the radial artery near the elbow serve as a significant risk factor for functional primary patency of RCAVF, with PSV above 59cm/s potentially associated with reduced RCAVF longevity. The unexpected finding reminds us to not only assess artery velocity adequacy but also pay attention to excessive peak systolic velocity, although further multicenter studies are needed for evaluation.
Funding sourcesThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors declare that they have no conflicts of interest.
We would like to express our gratitude to the esteemed colleagues in the Ultrasound Department at our hospital, as well as extend our appreciation to the nephrologists and nurses in the Nephrology Department within our institution.