Syndrome of inappropriate antidiuresis (SIAD) is an emerging problem in cancer patients and is responsible for one-third of cancer-related hyponatremia. SIAD can cause reduced quality of life, longer hospital admissions, and increased costs.1
Fluid restriction (FR) and increasing renal free water excretion are the basis of chronic cancer-related hyponatremia treatment. International guidelines put loop diuretics, urea, vaptans, or demeclocycline after FR, as first-line treatments.2,3
Urea had increased interest in the last years due to its effectiveness, and good safety profile.4 We hypothesized that urea is a good option in the treatment of SIAD in cancer patients.
We enrolled 11 cancer patients who were diagnosed with SIAD, from August 2021 until March 2022, that were treated with oral urea. We collected serum sodium (sNa) at baseline, 24h after starting treatment, and after that on basis of natremia evolution. We also record the time to reach sNa above 130mEq/L and 135mEq/L, the dose and duration of treatment and reported side effects.
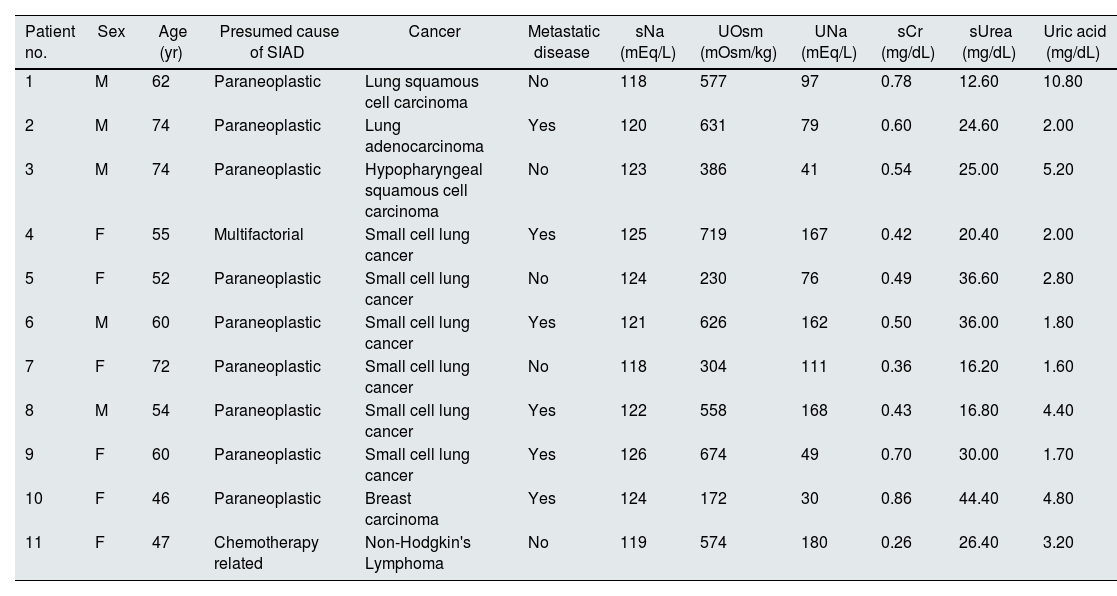
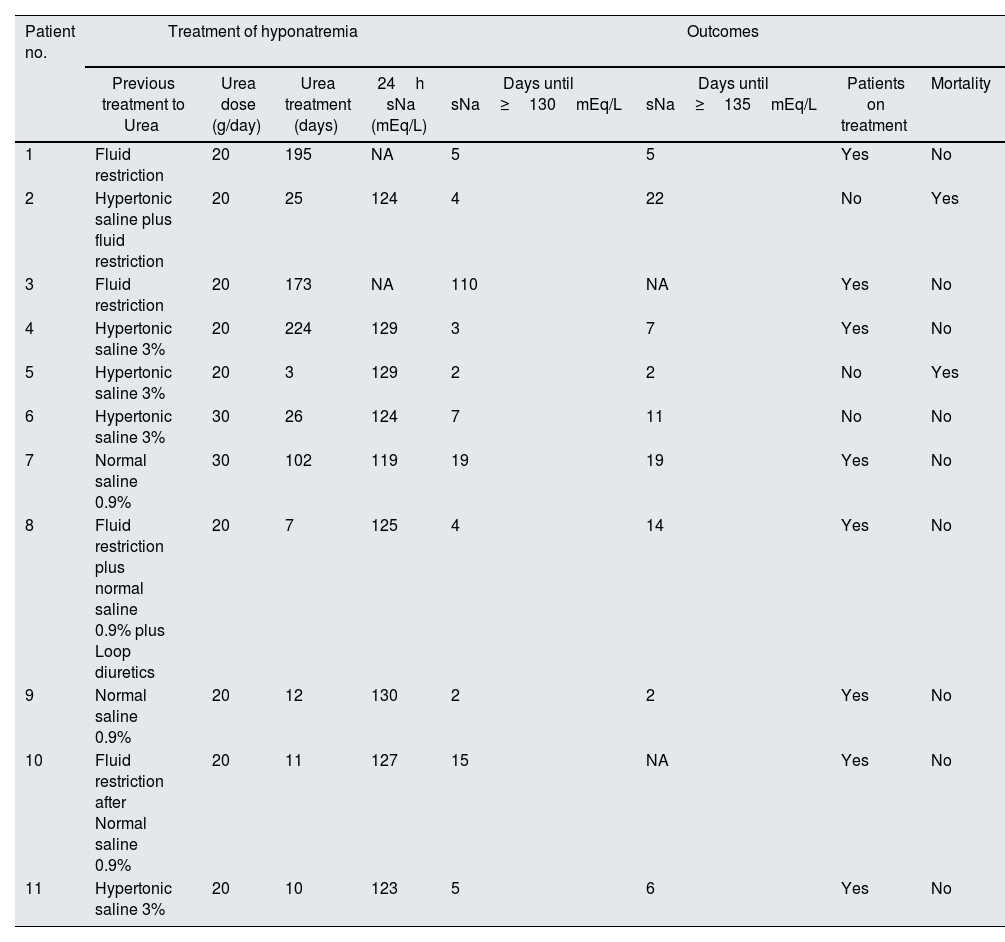
We observed that severe and chronic hyponatremia was present at admission in 82% of patients. Mean sNa was 121[±3]mEq/L and all have normal kidney function (Table 1). After 24h of treatment a mean increase of 3[±1]mEq/L occurred. The median time to reach sNa levels ≥130mEq/L and ≥135mEq/L were 5 (IQR 3–15, range 2–110) and 9 (IQR 4–20, range 2–60) days, respectively. Patients were treated with urea for a median of 25 days (IQR 10–178, range 3–224) (Table 2). In 4 patients we have records of urea distaste but not motivated discontinuation of treatment. Neither renal impairment, nor significant alterations of other electrolytes were detected. Mortality at end of study was 18% and no link to hyponatremia was identified.
Clinical and laboratory characteristics of patients with the Syndrome of inappropriate antidiuresis.
| Patient no. | Sex | Age (yr) | Presumed cause of SIAD | Cancer | Metastatic disease | sNa (mEq/L) | UOsm (mOsm/kg) | UNa (mEq/L) | sCr (mg/dL) | sUrea (mg/dL) | Uric acid (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 62 | Paraneoplastic | Lung squamous cell carcinoma | No | 118 | 577 | 97 | 0.78 | 12.60 | 10.80 |
| 2 | M | 74 | Paraneoplastic | Lung adenocarcinoma | Yes | 120 | 631 | 79 | 0.60 | 24.60 | 2.00 |
| 3 | M | 74 | Paraneoplastic | Hypopharyngeal squamous cell carcinoma | No | 123 | 386 | 41 | 0.54 | 25.00 | 5.20 |
| 4 | F | 55 | Multifactorial | Small cell lung cancer | Yes | 125 | 719 | 167 | 0.42 | 20.40 | 2.00 |
| 5 | F | 52 | Paraneoplastic | Small cell lung cancer | No | 124 | 230 | 76 | 0.49 | 36.60 | 2.80 |
| 6 | M | 60 | Paraneoplastic | Small cell lung cancer | Yes | 121 | 626 | 162 | 0.50 | 36.00 | 1.80 |
| 7 | F | 72 | Paraneoplastic | Small cell lung cancer | No | 118 | 304 | 111 | 0.36 | 16.20 | 1.60 |
| 8 | M | 54 | Paraneoplastic | Small cell lung cancer | Yes | 122 | 558 | 168 | 0.43 | 16.80 | 4.40 |
| 9 | F | 60 | Paraneoplastic | Small cell lung cancer | Yes | 126 | 674 | 49 | 0.70 | 30.00 | 1.70 |
| 10 | F | 46 | Paraneoplastic | Breast carcinoma | Yes | 124 | 172 | 30 | 0.86 | 44.40 | 4.80 |
| 11 | F | 47 | Chemotherapy related | Non-Hodgkin's Lymphoma | No | 119 | 574 | 180 | 0.26 | 26.40 | 3.20 |
F, female; M, male; NA, not applicable; sCr, serum creatine, sNa, serum sodium, UNa, urine sodium; UOsm, urine osmolality; sUrea, serum urea, yr, years.
Treatment parameters and outcomes.
| Patient no. | Treatment of hyponatremia | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|
| Previous treatment to Urea | Urea dose (g/day) | Urea treatment (days) | 24h sNa (mEq/L) | Days until sNa≥130mEq/L | Days until sNa≥135mEq/L | Patients on treatment | Mortality | |
| 1 | Fluid restriction | 20 | 195 | NA | 5 | 5 | Yes | No |
| 2 | Hypertonic saline plus fluid restriction | 20 | 25 | 124 | 4 | 22 | No | Yes |
| 3 | Fluid restriction | 20 | 173 | NA | 110 | NA | Yes | No |
| 4 | Hypertonic saline 3% | 20 | 224 | 129 | 3 | 7 | Yes | No |
| 5 | Hypertonic saline 3% | 20 | 3 | 129 | 2 | 2 | No | Yes |
| 6 | Hypertonic saline 3% | 30 | 26 | 124 | 7 | 11 | No | No |
| 7 | Normal saline 0.9% | 30 | 102 | 119 | 19 | 19 | Yes | No |
| 8 | Fluid restriction plus normal saline 0.9% plus Loop diuretics | 20 | 7 | 125 | 4 | 14 | Yes | No |
| 9 | Normal saline 0.9% | 20 | 12 | 130 | 2 | 2 | Yes | No |
| 10 | Fluid restriction after Normal saline 0.9% | 20 | 11 | 127 | 15 | NA | Yes | No |
| 11 | Hypertonic saline 3% | 20 | 10 | 123 | 5 | 6 | Yes | No |
sNa=plasma sodium; sNa24=serum sodium at 24h of treatment; NA=not available.
We endorse that in SIAD patients is particularly relevant to understand the whole-body volume regulation. In early SIAD stages, water retention is induced by antidiuretic stimulus, but with its continuity, natriuresis occurs to regulate de volume distribution between extracellular and intracellular space.5
The imbalance between water and sodium makes urea an interesting treatment option, because its effects not only involve osmotic diuresis but also the urinary sodium excretion.5
Urea is stored in inner medulla and is responsible for 50% of solutes in this segment. The urea transporters (UT), who are distributed in thin limb of loop of the Henle (UT-A2) and in inner medullary collecting duct (UT-A1/3), are responsible for this urea stored and recycling process.5,6
Urea is secreted in the initial portion of thick ascending limb of Henle, promoting an increase in intratubular urea concentration by to 110% of initial filtered load. Next segments are relatively impermeable to urea until the outer medullary collecting duct. At terminal inner medullary collecting duct (IMCD) permeability will increase and only 50% of urea remain in the lumen. In the presence of ADH, 20% is absorbed through the UT-A1 transporters in apical membrane in IMCD cells and exits this cell via UT-A3 in basolateral membranes, which leads to a total excreted load of 30%.6
Previous studies suggested that UT-A1/A3 are saturable transporters.7 This may explain why an amount of urea reaching this region is not transported to the medullary region, remaining in the tubular lumen, thus increasing osmotic diuresis.
Recent study working groups have include urea as second-line treatment strategy for managing SIAD.2,3 In cancer patients removing the primary cause sometimes is not possible and additional treatment are needed. These patients have a higher risk of hypovolemic status and FR institution is difficult in needs of chemotherapeutic infusions.8
In a cohort of cancer patients with SIAD treated with urea, 40% of the patients reached sodium above 130mEq/L during the first 24h of treatment and 86.1% reached eunatremia during the first month.9 In our study the time required to attain sodium above 130mEq/L varied–median of 5 (IQR 3–15, range 2–110) days, but was achieved in all patients. The eunatremia was reached in 64% (n=7) patients.
Urea was generally well tolerated, despite 50% of patients refers unpleasant taste it was not enough to interrupt the treatment.2,3 Demyelination syndrome has not been described in patients treated with urea, moreover it can even have a cerebral protective effect when overcorrection occurs.10
Other SIAD treatment option includes vaptans. The first study that compared vaptans to urea concluded that these patients could be treated with the same safety, efficacy and tolerance with either drugs.4 However, European guidelines do not encourage the use of vaptans in SIAD.2
Our study reinforces that oral urea in chronic hyponatremia cancer patients appears to be effective, safe, and well tolerated, with no serious adverse events reported. Urea can be used for a long period of time in ambulatory patients, becoming a therapeutic approach in cancer patients, which can possibly reduce hospitalizations, improve quality of life and survival with a good cost-effectiveness profile.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestNone declared.