Multiple myeloma (MM) is a clonal plasma cell malignancy characterized by excessive production of immunoglobulins and/or free light chains (FLCs). It predominantly affects individuals aged 65–74, with approximately 35,000 new cases annually in the U.S.1 Renal complications are common, affecting 20–50% of patients at diagnosis, varying between FLC deposition, amyloidosis, hypercalcemia or direct tubular toxicity.1 Acute kidney injury (AKI) in this context is associated with high mortality and worsened prognosis.2
Management involves renal supportive care like aggressive hydration, nephrotoxin avoidance, and dialysis as needed; and chemotherapy aiming to reduce monoclonal protein burden, typically including proteasome inhibitors (e.g., bortezomib), immunomodulatory agents, steroids, and recently daratumumab.1
Because chemotherapy has a delayed effect, renal damage can progress before adequate FLC clearance is achieved. This has led to interest in extracorporeal therapies for cast nephropathy, including plasmapheresis, high cut-off filters, and hemoadsorption (HA).
Hemoadsorption is an extracorporeal technique designed to remove solutes, cells, or toxins with properties—such as size, protein binding, or lipophilicity—that limit their clearance by diffusion or convection.3 This case report highlights the first use of the HA380 hemoadsorption cartridge in MM-related cast nephropathy. The HA380 contains a neutral macroporous resin and targets molecules between 10 and 60kDa, making it particularly suited for removing FLCs. Originally developed for critical conditions like sepsis, trauma, pancreatitis, burns, COVID-19, and acute respiratory distress syndrome (ARDS);4 the cartridge can be integrated with renal replacement therapy (RRT) to enhance clearance.
Case reportA 76-year-old woman presented with a one-year history of weight loss, asthenia, anorexia, and foamy urine. On emergency admission, she had dyspnea, orthopnea and edema up to the thighs. Laboratory tests revealed anemia (hemoglobin 7.9g/dL), elevated creatinine (2.6mg/dL; baseline 0.48mg/dL), and cystatin C (3.43mg/L). Urinalysis showed albuminuria (1980mg/g) and a creatinine-protein ratio of 25g/g. Further workup identified a lambda free light chain (FLC) monoclonal gammopathy (1230mg/dL). Urinary immunofixation confirmed lambda FLC excretion.
During hospitalization, she developed atrial flutter (HR 170bpm) and hemodynamic instability, requiring intensive care unit admission. Within twenty four hours, her condition worsened: worsening AKI (creatinine 3.4mg/dL), cholestasis (ALP 234, GGT 219), hypervolemia unresponsive to diuretics, and respiratory failure. A chest X-ray revealed bilateral pulmonary congestion and right-sided pleural effusion. Echocardiography showed severe tricuspid regurgitation, biventricular dysfunction, IVC dilation (22mm, no variability), and septal thickness of 11mm. She also developed severe metabolic and lactic acidosis (pH 7.18, HCO3− 11.4mmol/L, lactate 8.9mmol/L).
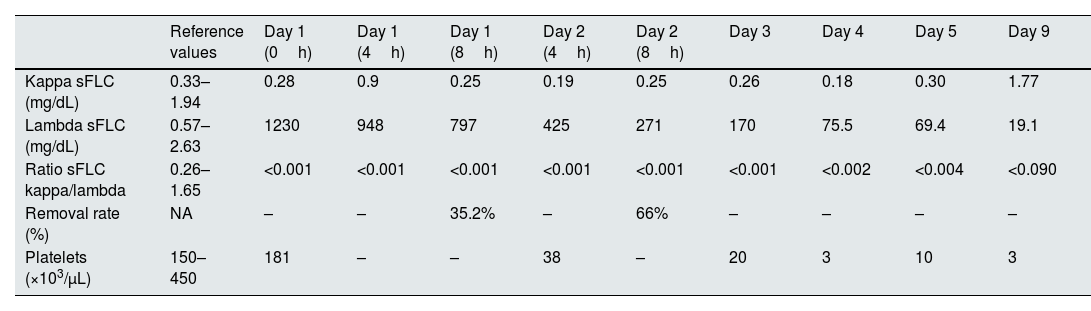
A presumptive MM diagnosis was made, and treatment was started with dexamethasone, bortezomib, and continuous veno-venous hemodiafiltration (CVVHDF) combined with the HA380 hemoadsorption cartridge, without anticoagulation. Two 8-hour sessions were performed (Table 1). Lambda FLC levels fell from 1230 to 797mg/dL after the first session, then to 271mg/dL after the second. Removal rates were 35.2% and 66%, respectively. Bortezomib was given after the first session.
Evolution of serum free light chains levels, removal rate and platelets during the treatment timeline.
| Reference values | Day 1 (0h) | Day 1 (4h) | Day 1 (8h) | Day 2 (4h) | Day 2 (8h) | Day 3 | Day 4 | Day 5 | Day 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Kappa sFLC (mg/dL) | 0.33–1.94 | 0.28 | 0.9 | 0.25 | 0.19 | 0.25 | 0.26 | 0.18 | 0.30 | 1.77 |
| Lambda sFLC (mg/dL) | 0.57–2.63 | 1230 | 948 | 797 | 425 | 271 | 170 | 75.5 | 69.4 | 19.1 |
| Ratio sFLC kappa/lambda | 0.26–1.65 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.002 | <0.004 | <0.090 |
| Removal rate (%) | NA | – | – | 35.2% | – | 66% | – | – | – | – |
| Platelets (×103/μL) | 150–450 | 181 | – | – | 38 | – | 20 | 3 | 10 | 3 |
sFLC – serum free light chains; NA – non applicable.
The patient developed thrombocytopenia, remaining the etiology inconclusive. Notably, thrombocytopenia continued to worsen even after discontinuation of the treatment. No other adverse effects were observed.
Despite all measures, she died from refractory cardiogenic shock nine days after ICU admission.
DiscussionThe theory that rapidly removing FLCs could improve MM-associated AKI began to be tested with plasma exchange (PE). Early studies were encouraging, but a 2005 randomized trial (97 patients; 58 received PE) showed no benefit in mortality, dialysis dependence, or renal recovery at 6 months. PE remains controversial due to conflicting data and risks like loss of clotting factors and bleeding.5
High-cutoff hemodialysis (HCO-HD) was introduced as a more targeted method, since FLCs (22.5–45kDa) fall within its membrane range (cutoff: 45–60kDa).6 Its efficacy was assessed in two randomized trials:
- •
MYRE trial (98 patients with biopsy-proven cast nephropathy): HCO-HD improved FLC clearance but not survival or dialysis independence at 3 months7;
- •
EuLITE trial (90 patients, bortezomib-based therapy): No renal recovery benefit; higher mortality and more serious adverse events with HCO-HD. Hypoalbuminemia was common, often requiring 20% albumin supplementation.8
We evaluated hemadsorption with HA380 as a targeted method for FLC removal in MM-AKI. It achieved rapid FLC reduction comparable to HCO-HD, with minimal off-target protein loss, suggesting a big potential as an early intervention. Though our patient remained dialysis-dependent, the approach was well-tolerated overall.
Despite promising results in reducing FLC levels, several concerns persist: possible overestimated clearance, since only ∼20% of FLCs are intravascular1; impact on chemotherapy because lower FLCs may reduce bortezomib efficacy8; and monitoring difficulty since sFLC levels guide therapy and extracorporeal removal may interfere.1
Thrombocytopenia was the only observed side effect and it is a known, typically transient reaction associated with HA380, likely due to platelet aggregation9. However, in this case, thrombocytopenia persisted even after discontinuation of treatment, suggesting it was more likely related to the patient's underlying inflammatory state.
Further studies, including randomized trials, are needed to assess HA cartridge efficacy in FLC removal, impact on renal recovery and mortality, and potential adverse effects, but it seems to be a promising technique.
FundingThis study received no external funding.
Conflicts of interestThe authors declare no conflicts of interest.