Adult Still's disease (ASD) is a systemic inflammatory disease of unknown etiology. Renal involvement in ASD is a rare and rarely reported manifestation in the literature. This report presents an atypical case of ASD with acute renal failure.
A 39-year-old male with arterial hypertension treated with Ramipril 10mg was admitted to the Emergency Department (ED) with a fever of up to 39°C, right laterocervical edema, dysphagia, and odynophagia the last four days. He started treatment with Amoxicillin-Clavulanate before consultation in the ED. The patient did not report country outings or recent trips. He highlighted an infestation of rodents in his building. The patient denied consuming alcohol, tobacco and other drugs, or mushrooms, herbal products, or other substances that could have caused liver damage.
Physical examination revealed mild jaundice, a temperature of 38°C, non-adhered right laterocervical lymphadenopathy of 1–2cm, enlarged tonsils with discrete whitish plaques on their surface, and a macular and itchy rash on the upper trunk and forearms. Laboratory tests at ED showed: procalcitonin 1.10ng/ml, urea 69mg/dL, creatinine 2.79mg/dL, sodium 135mmol/L, potassium 4.5mmol/L, chlorine 98mmol/L, GPT 114U/L, CK 233U/L, Amylase 35U/L, C reactive protein (CRP) 286.9mg/L, leukocytes 15,430/mcL (neutrophils 13,740/mcL), prothrombin activity 63%. Abdominal ultrasound and chest X-ray were normal. The patient presented the day after admission: total bilirubin 9mg/dL, direct bilirubin 8.4mg/dL, GGT 143IU/L, and Alkaline Phosphatase 267IU/L. Leukocytosis reached 33,000/mcL with 92% polymorphonuclear cells. The rise in ferritin levels was remarkable with 4.104 mcg/mL. During admission, the patient presented a rapid onset acute renal failure with anuria. The renal function worsened with a creatinine of 10.36mg/dL. This clinical course led to hemodialysis therapy from the second day of admission.
Immunoglobulin A level was 479mg/dL. C3, C4, Antistreptolysin O, antinuclear antibodies, nuclear extractible antigen antibodies, anti-DNA antibodies, neutrophil anti-cytoplasmic antibodies were normal or negative. Interleukin-6 was 148pg/mL. Plasma protein electrophoresis was compatible with an acute inflammatory process.
Nasopharyngeal swab for Polymerase Chain Reaction for SARS-CoV2 and pharyngeal swab for Streptococcus pyogenes were both negative. Urine and blood cultures were negative. Serologic studies ruled out acute infection by Cytomegalovirus, Epstein-Bar virus, Measles, Q fever, Leptospira, hepatitis A and B viruses, Human Immunodeficiency Virus, and parvovirus B19. A computed tomography (CT) scan showed a slightly enlarged 15-cm spleen. A Positron Emission Tomography-CT scan was normal.
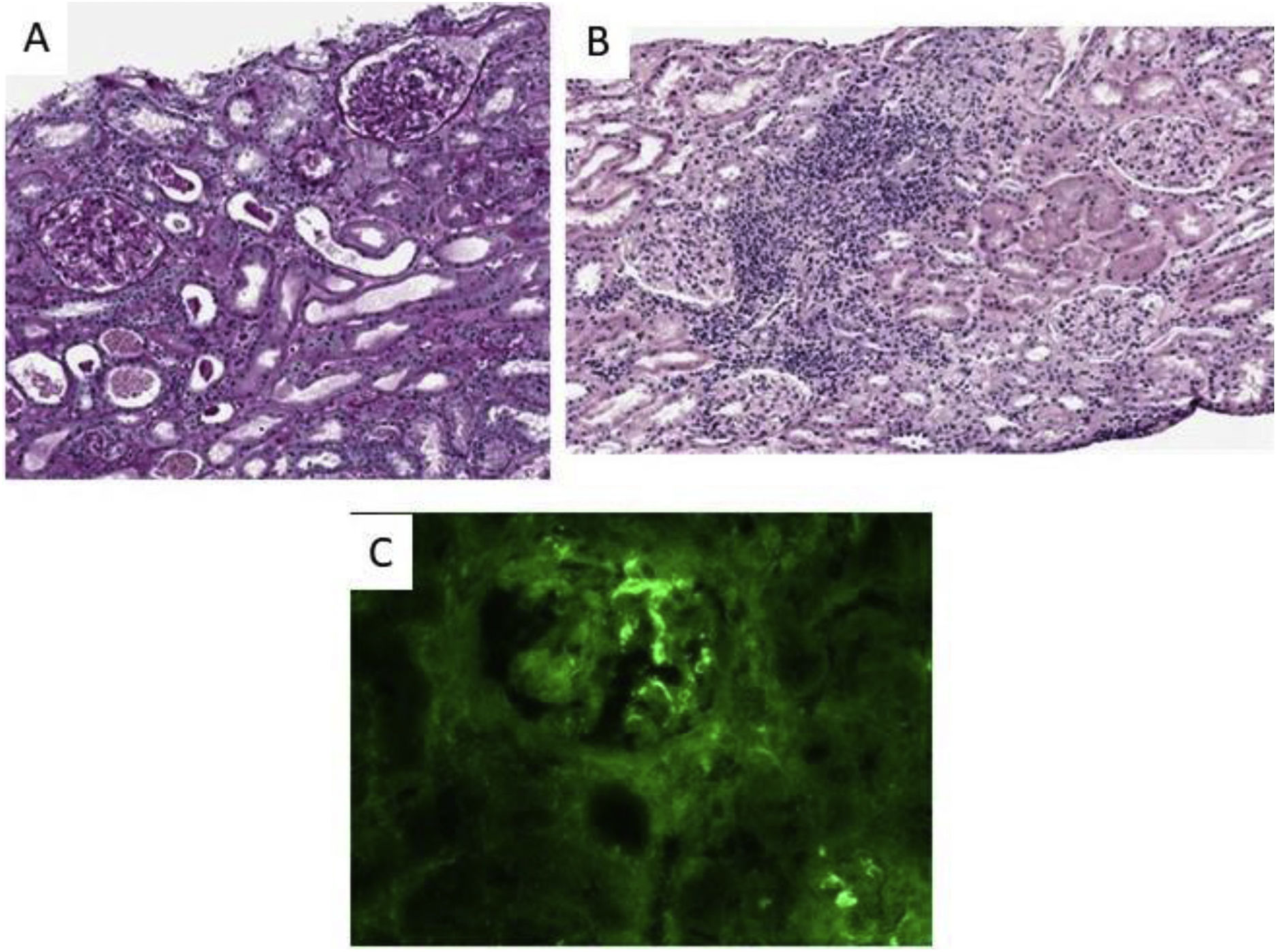
Finally, a renal biopsy showed an acute tubulointerstitial inflammatory infiltrate with some ruptured tubule and isolated eosinophils associated with a dense lymphoplasmacytic infiltrate and a mesangial deposit anti-IgA (++) and minimal anti-C3, all of which was compatible with IgA mesangial nephropathy and acute tubulointerstitial nephritis (Fig. 1). However, there was an absence of intratubular erythrocyte casts or crescents in the biopsy.
Our patient meets the criteria of both Yamaguchi and Fautrel for presumptive diagnosis of ASD (Table 1). Corticosteroid treatment was started initially with Prednisone 60mg/day, and subsequently escalating to 500mg of 6-Methylprednisolone daily associated with Anakinra at 100mg/week, causing a rapid and progressive improvement in the general condition and rash, a decrease in the acute phase reactants and leukocytosis, and an improvement in his renal function. The patient recovered normal diuresis and was discharged with a creatinine of 2.04mg/dL with proteinuria of 25mg/dL in basic urine examination. After discharge from the hospital, the patient fully recovers renal function with a creatinine of 1mg/dL.
Diagnostic criteria of ASD.
| Criteria of Yamaguchi et al. [4]a | |
|---|---|
| Major criteria:- Fever≥39°C≥1 week- Arthralgias/Arthritis for 2 or more weeks- Typical evanescent rash- Leukocytosis≥10,000/mm3 with ≥80% polymorphonuclear | Minor criteria:- Odynophagia- Lymphadenopathy- Splenomegaly- Alteration of liver function tests- Rheumatoid factor and ANA negatives |
| Criteria of Fautrel et al. [5]b | |
|---|---|
| Major criteria:- Spiking fever≥39°C- Arthralgia- Transient skin rash- Odynophagia- Polymorphonuclear cells>80%- Glycated ferritin≤20% | Minor criteria:- Maculopapular rash- Leukocytosis>10,000/mm3 |
Since there are currently no specific diagnostic tests, ASD diagnosis is usually based on the clinical recognition of the entity, always ruling out other possible etiologies such as infections or neoplasms. Among the criteria used for its diagnosis are the Yamaguchi criteria1 and the Fautrel criteria2 (Table 1). Elevated procalcitonin, C-reactive protein, and leukocytosis with neutrophilia could lead the clinician to suspect other etiologies, especially infectious, and diagnostic delay. Serum ferritin or cytokines, such as IL-6, can help diagnosis. The combination that has shown the greatest diagnostic accuracy was serum ferritin>1000mcg/L or values five times above the upper limit of normal, together with a fraction of glycated ferritin<20%. Its sensitivity and specificity are 70.5% and 92.9%, respectively.3,4
Still's disease can present more atypical manifestations, such as acute renal failure of rapid onset. Entities associated in previous literature were Amyloidosis,5 Thrombotic Microangiopathy,6 IgA Glomerulonephritis,7 and more recently, cases of collapsing glomerulopathy.8 However, despite being described in reviews on Still's disease,9 only one case of interstitial nephritis has been found reported in the consulted bibliography.10
Therefore, it is essential to include ASD in the differential diagnoses of acute renal failure accompanied by analytical markers such as elevated IL-6 or ferritin of up to five times its standard value. Early treatment of this disease can lead to the complete recovery of the patient's previous renal function.
Informed consentThe patient reported here has provided written informed consent for publication of this case report.
FundingThis work has no funding.
Conflict of interestNone.