Sjögren's syndrome (SS) is an autoimmune disorder characterized by lymphocytic infiltration of the exocrine glands. Approximately half of the patients will present with systemic symptoms, and around 5% will develop lymphoma.1,2
Renal involvement is one of the systemic complication that occurs in 5%–14% of cases, with chronic tubulointerstitial nephritis being the most prevalent SS-associated nephropathy. Glomerular involvement is uncommon but potentially serious and it is associated with cryoglobulinemic vasculitis in more than half of the cases.3,4
However, the differential diagnosis of renal manifestations in SS is broad, taking into account the possible association with other immune-mediated diseases or lymphoproliferative disorders.3 We present the case of a patient with SS and an initial suspicion of chronic tubulointerstitial nephritis, which required a multidisciplinary approach to reach a diagnosis.
The patient was a 62-year-old woman with a history of SS with systemic involvement (lymphoid interstitial pneumonia, leukocytoclastic vasculitis, and immune thrombocytopenia) who was admitted to the hospital with abdominal pain, vomiting, and diarrhea of 2 months' duration.
Physical examination revealed ascites and edema in the lower extremities with nonpalpable purpura. Laboratory tests revealed acute deterioration of renal function, elevated rheumatoid factor (62.6 IU/mL), hypoalbuminemia, and hypocomplementemia (C3: 26.8 mg/dL, C4: 1.7 mg/dL), and an elevated urine protein-to-creatinine ratio (1071 mg/g).
The computed tomography scan showed hepatomegaly, splenomegaly, and thoracoabdominal lymphadenopathies with no pathological uptake on positron emission tomography. Gastrointestinal endoscopies with biopsies of the stomach, ileum, and colon revealed no abnormalities.
On the fourth day of admission, the patient developed severe anemia and thrombocytopenia (4000 platelets/µL), with no evidence of hemolysis or thrombotic microangiopathy. She was receiving pulses of methylprednisolone and subcutaneous romiplostim. Two weeks later, she experienced worsening renal function with increased proteinuria and volume overload refractory to diuretics, requiring renal replacement therapy.
Given the patient's clinical course, it was suspected a glomerular disease, and a differential diagnosis was made between cryoglobulinemic vasculitis and monoclonal gammopathy. A multidisciplinary approach, including hematology and nephrology, was initiated with plasmapheresis and intravenous immunoglobulins. The response was suboptimal, so a single dose of rituximab was administered.
The workup was completed by measuring serum β2-microglobulin (13.2 mg/L), ADAMTS13 activity (>10%), antiphospholipid antibodies (negative), and cryoglobulins (repeatedly negative). Serum and urine electrophoresis and immunofixation detected a monoclonal IgG lambda peak in serum and kappa light chain peaks in urine, with a pathological kappa/lambda ratio (−8.59). The peripheral blood smear was normal, and the bone marrow biopsy identified 8% plasma cells (CD138/kappa).
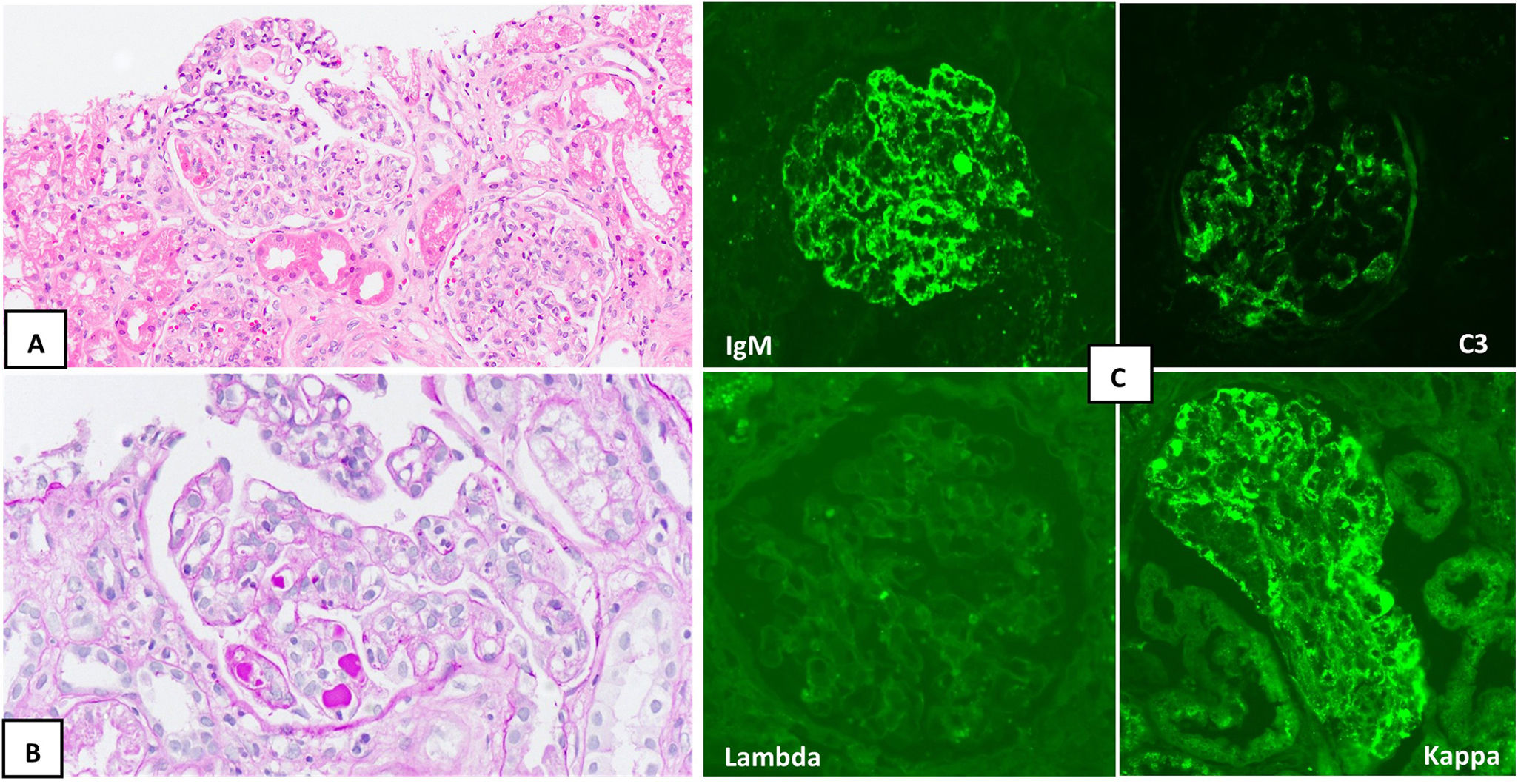
After resolution of the thrombocytopenia, a renal biopsy revealed membranoproliferative glomerulonephritis with focal pseudo thrombi and kappa light chain restriction (IgM/C3/kappa) (Fig. 1). Based on these findings, a diagnosis of monoclonal gammopathy of renal significance (MGRS) was made, although an associated cryoglobulinemic vasculitis could not be ruled out.
Renal biopsy. A) Two glomeruli with increased mesangial cellularity are identified (hematoxylin-eosin, ×20). B) Focal pseudothrombi and double-contour images (PAS, ×20) are recognized. C) Direct immunofluorescence shows positivity for IgM (top left) and C3 (top right), with a predominance of kappa (bottom right) versus lambda (bottom left).
Renal function and proteinuria progressively improved, leading to the patient's discharge, which remained stable during outpatient follow-up.
Renal involvement in SS occurs through two immunopathological mechanisms: infiltration of the tubular epithelium by activated lymphocytes and immune complex-mediated glomerulopathy. The former usually produces chronic tubulointerstitial nephritis, and the latter membranoproliferative glomerulonephritis secondary to cryoglobulinemic vasculitis.
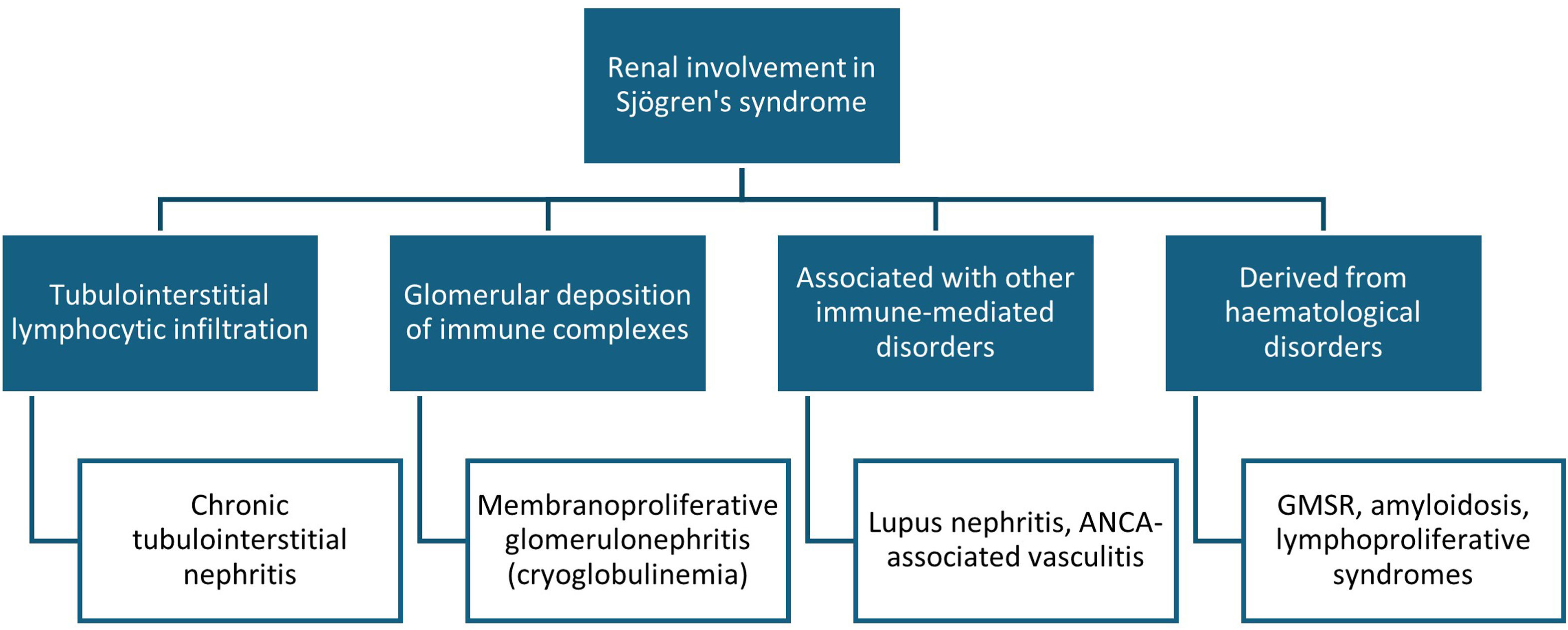
However, in SS, renal manifestations may occur secondary to other immune-mediated disorders or as a consequence of hematological complications arising from chronic B cell activation3,5 (Fig. 2).
Renal involvement in Sjögren's syndrome (SS). Classically, two types of renal manifestations are distinguished in SS: tubulointerstitial nephritis and immune complex deposition glomerulopathy. However, renal involvement in SS also includes nephritis secondary to other associated immune-mediated diseases (such as systemic lupus erythematosus) or renal complications arising from lymphoproliferative disorders (such as monoclonal gammopathy of renal significance [MGRS] or renal lymphoma). ANCA: antineutrophil cytoplasmic antibodies.
It is estimated that 20% of patients with SS have monoclonal gammopathy of uncertain significance (MGUS), which is associated with a higher prevalence of hypergammaglobulinemia, cryoglobulinemia, and hematological malignancies. The coexistence of SS and MGUS presents a worse prognosis, so periodic serum immunoelectrophoresis is recommended in patients with SS.6
In recent years, there have been described cases of MGRS associated with SS, mainly in the form of a monotypic plasma cell infiltrate in the renal interstitium.7 In a recently published case series of mixed cryoglobulinemic glomerulonephritis, all four patients included were found to have MGUS with IgM-kappa paraprotein. Interestingly, three of the four were diagnosed with SS.8
The simultaneous presentation of SS, membranoproliferative glomerulonephritis, and MGUS is a complex scenario. Our case is analytically and histologically compatible with MGRS. However, the systemic and multiorgan involvement is best explained by incomplete cryoglobulinemic vasculitis, as she presented with typical clinical and pathological manifestations in the absence of detectable cryoglobulins in serum.9,10 Corticosteroids and anti-CD20 antibodies are used in both entities, which would explain our patient's improvement after starting rituximab.
In conclusion, MGRS may be underdiagnosed and represents another manifestation of the lymphoproliferative spectrum of SS. In a patient with MGUS and SS with renal involvement, the possibility of MGRS should be suspected since early initiation of treatment could improve the prognosis.
FundingThis article has no sources of funding.
The authors declare that they have no conflicts of interest.



![Renal involvement in Sjögren's syndrome (SS). Classically, two types of renal manifestations are distinguished in SS: tubulointerstitial nephritis and immune complex deposition glomerulopathy. However, renal involvement in SS also includes nephritis secondary to other associated immune-mediated diseases (such as systemic lupus erythematosus) or renal complications arising from lymphoproliferative disorders (such as monoclonal gammopathy of renal significance [MGRS] or renal lymphoma). ANCA: antineutrophil cytoplasmic antibodies. Renal involvement in Sjögren's syndrome (SS). Classically, two types of renal manifestations are distinguished in SS: tubulointerstitial nephritis and immune complex deposition glomerulopathy. However, renal involvement in SS also includes nephritis secondary to other associated immune-mediated diseases (such as systemic lupus erythematosus) or renal complications arising from lymphoproliferative disorders (such as monoclonal gammopathy of renal significance [MGRS] or renal lymphoma). ANCA: antineutrophil cytoplasmic antibodies.](https://static.elsevier.es/multimedia/20132514/0000004500000007/v2_202512080931/S2013251425001014/v2_202512080931/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w94GCRvdQBB6xyQjMrWMzrts=)



