The knowledge about the acute kidney injury (AKI) incidence in patients with coronavirus disease 2019 (COVID-19) can help health teams to carry out a targeted care plan. This study aimed to determine the AKI incidence in patients hospitalized with COVID-19.
MethodsThe electronic search covered research published until June 20, 2020, and included five databases, PubMed, Embase, Web of Science, Scopus, and Lilacs (Latin American and Caribbean Health Sciences Library). Eligible studies were those including data from AKI occurrence in adult patients hospitalized with COVID-19. The primary outcome was AKI incidence, and the secondary outcome assessed was the AKI mortality. Additionally, the estimated incidence of renal replacement therapy (RRT) need also was verified. Using a standardized form prepared in Microsoft Excel, data were extracted by two independents authors, regarding the description of studies, characteristics of patients and clinical data on the AKI occurrence.
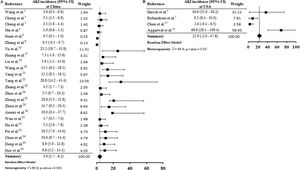
ResultsWe included 30 studies in this systematic review, of which 28 were included in the meta-analysis. Data were assessed from 18.043 adult patients with COVID-19. The AKI estimate incidence overall and at the ICU was 9.2% (4.6–13.9) and 32.6% (8.5–56.6), respectively. AKI estimate incidence in the elderly patients and those with acute respiratory disease syndrome was 22.9% (−4.0–49.7) and 4.3% (1.8–6.8), respectively. Patients with secondary infection, AKI estimate incidence was 31.6% (12.3–51.0). The estimate incidence of patients that required RRT was 3.2% (1.1–5.4) and estimate AKI mortality was 50.4% (17.0–83.9).
ConclusionThe occurrence of AKI is frequent among adult patients hospitalized with COVID-19, and affects on average, up to 13.9% of these patients. It is believed that AKI occurs early and in parallel with lung injury.
El conocimiento de la incidencia de lesión renal aguda (LRA) en pacientes con enfermedad por coronavirus 2019 (COVID-19) puede ayudar a los equipos de atención médica a llevar a cabo un plan de atención específico. Este estudio tuvo como objetivo determinar la incidencia de LRA en pacientes hospitalizados con COVID-19.
MétodosLa búsqueda electrónica cubrió la investigación publicada hasta el 20 de junio del 2020 e incluyó 5 bases de datos: PubMed, Embase, Web of Science, Scopus y Lilacs (Biblioteca de Ciencias de la Salud de América Latina y el Caribe). Los estudios elegibles fueron aquellos que incluyeron datos sobre la aparición de LRA en pacientes adultos hospitalizados con COVID-19. El resultado primario fue la incidencia de LRA y el resultado secundario evaluado fue la mortalidad por LRA. Además, también se verificó la incidencia estimada de necesidad de terapia de reemplazo renal (TRR). Mediante un formulario estandarizado elaborado en Microsoft Excel, los datos fueron extraídos por 2 autores independientes, haciendo referencia a la descripción de los estudios, las características de los pacientes y los datos clínicos sobre la ocurrencia de LRA.
ResultadosEn esta revisión sistemática se incluyeron 30 estudios, de los cuales 28 se incluyeron en el metaanálisis. Se evaluaron los datos de 18.043 pacientes adultos con COVID-19. La incidencia estimada de LRA en general y en la UCI fue del 9,2% (4,6-13,9) y del 32,6% (8,5-56,6), respectivamente. La incidencia estimada de LRA en pacientes ancianos y pacientes con síndrome de enfermedad respiratoria aguda fue del 22,9% (–4,0-49,7) y del 4,3% (1,8-6,8), respectivamente. En pacientes con infección secundaria, la incidencia estimada de LRA fue del 31,6% (12,3-51,0). La incidencia estimada de pacientes que requirieron TRR fue del 3,2% (1,1-5,4) y la mortalidad estimada por LRA fue del 50,4% (17,0-83,9).
ConclusiónLa ocurrencia de LRA es frecuente en pacientes adultos hospitalizados con COVID-19 y afecta, en promedio, hasta al 13,9% de estos pacientes. Se cree que la LRA ocurre temprano y en paralelo con la lesión pulmonar.
Started in December 2019, after setting in Wuhan, China, the SARS-CoV-2 infection, responsible to the illness officially nominated as coronavirus disease 2019 (COVID-19), quickly spread to other regions, affecting several countries worldwide and, at the end of the 2020, on 27 December, the global number of infected and killed individuals by COVID-19 have been over 79.2 and 1.7 million, respectively.1 Frequent manifestations of COVID-19 include characteristic symptoms of an acute respiratory illness, as fatigue and dry cough, but may also occur others signs and symptoms as dyspnea, whereas acute respiratory distress syndrome (ARDS) is the main pulmonary complication.2 As well as the lung, other organs can also be affected, such as the kidney and heart.2–6
Cheng et al.3 analyzed the occurrence of renal abnormalities in patients with COVID-19 from a Wuhan hospital and observed higher rate proteinuria, elevated serum creatinine and elevated blood urea nitrogen (BUN). The authors reported that the acute kidney injury (AKI) incidence was 5.1% and this complication was significantly associated with worse prognosis. In other large study, involving 5499 patients with COVID-19 from 13 hospitals in the New York metropolitan area,4 the AKI incidence was 36.6% and, just like in the Chinese study, the AKI occurrence in the North American patients also was associated with worse outcomes. In addition, the risk factors associated with AKI in this study were advanced age, comorbidities, need for ventilation and drug-associated.
Several studies report the occurrence of AKI and others adverse kidney events in hospitalized patients with COVID-19.2–10 However, the involvement of kidney and the epidemiological burden of AKI in patients with COVID-19 is still unclear and needs to be better studied.2–5 Therefore, this study aimed to determine the AKI incidence in patients hospitalized with COVID-19. The results of this systematic review will help healthcare teams to carry out a care plan with greater attention to the kidney function of patients with COVID-19.
MethodStudy registrationThe report of this systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist11 and the protocol was recorded on the International Prospective Register of Systematic Reviews (PROSPERO – CRD42020187355).
Eligibility criteriaInclusion criteriaA systematic search was performed to identify studies that reported data from AKI occurrence in adult patients hospitalized with COVID-19. The electronic search occurred between covered research published until June 20, 2020, and included five databases, PubMed, Embase, Web of Science, Scopus, and Lilacs (Latin American and Caribbean Health Sciences Library). In addition, to cover the gray literature, we also performed a manual search by checking the list of references of the reviews found and of the eligible studies (cross-reference). The keywords used were “COVID-19” and “acute kidney injury” and “incidence” or “mortality”, as well as its entry terms. The databases and the gray literature were accessed between June 4 and 8, 2020. The search strategy and the question of this study were structured according to the acronym CoCoP (Condition, Context, Population), of which (Co) was AKI incidence as primary outcome and AKI mortality as secondary outcome; (Co) hospital context; (P) adult patients with COVID-19 (S1 File).
Searches were performed in English, Portuguese, and Spanish. Data were selected in English, Portuguese, and Spanish. Data were also selected from adult (≥ 18 years) patients, hospitalized, with confirmed diagnosis of COVID-19 and that reported data of AKI occurrence and other clinical data associated. Additionally, to avoid selection bias of pairs of studies have been included that use data collected by the same working groups3–5,7 in the same period, we verified the possibility that they were the same patients by making personal contact with the authors of the studies and we were not told that they were the same patients.
Exclusion criteriaThus, studies were excluded that: (1) do not report at least the AKI incidence; (2) included data from children and adolescents; (3) with a very small sample (≤ 10 patients); (4) do not report original data, such a editorials, comments, and other reviews; (5) case reports; (6) with exclusive data from patients with chronic kidney disease (CKD) and/or kidney transplant; (7) study with duplicate data.
Information sources and study selectionTwo independent authors (RP, TVAL) performed the search in the databases and assessed the eligibility of the studies, first reading the titles and abstract and, then reading in full the studies with potential for inclusion in this systematic review. Disagreements between the two researchers were resolved by consensus and, in the absence of consensus, a third researcher (ARSC) was consulted. In addition, the list of studies in the final selection, and included in this systematic review was also approved by a fourth researcher (LABP). Duplicate publications were excluded with the support of a reference manager.12
Data collection processUsing a standardized form prepared in Microsoft Excel, data were extracted by two independents authors (RP, TVAL), regarding the description of studies, characteristics of patients and clinical data on the AKI occurrence, as described below:
- •
Study description: code (reference number), authors, year, journal, article title, country, study type, length of data collection, number of hospitals, number of patients enrolled and number of patients in the Intensive Care Unit (ICU).
- •
Patients’ characteristics: mean age, male gender rate, time between symptom onset and hospitalization, complications (ARDS, acute cardiac injury (ACI), secondary infection, sepsis, or shock), in-hospital mortality rate.
- •
AKI occurrence: definition, AKI overall incidence, AKI incidence in ICU, need of Renal Replacement Therapy (RRT) and AKI mortality. Considering that incidence rates include only the first occurrence, in order to collect only incidence data, cases registered as AKI de novo were not considered.
The risk of bias of selected studies was assessed by two independent authors (RP, TVAL), using the Checklist for Analytical Cross-Sectional Studies, a Joanna Briggs Institute's critical appraisal tool.13 The studies were considered with high, moderate, or low risk of bias when reached a positive (+) score of up to 49% (less than four positive assessments), score of 50–69% (between four and five positive assessments), and score of more than 70% (six or more positive assessments), respectively. The risk of publication bias was assessed using funnel plots. All studies meeting the inclusion criteria at the quantitative analysis were assessed for methodological quality.
Statistical analysisThe primary outcome was AKI incidence, being determined the estimated mean and 95% confidence interval (95% CI). The pooled AKI incidence overall was determined, as well as in the ICU and for the following different subgroups: (1) according to the geographic localization of studies; (2) in the elderly patient (with median or mean age>60 years); (3) in the patients with early hospitalization (with median or mean of time between symptom onset and hospitalization≤7 days); (4) patients with following complications: (4.1) ARDS; (4.2) ACI; (4.3) with secondary infections without sepsis/shock; (4.4) in the patients with sepsis/shock (with or without other complications). The secondary outcome assessed was the AKI mortality, also determined by the estimated mean and 95% CI. Additionally, the estimated incidence of RRT need was also verified.
To identify possible factors associated with a higher AKI incidence, the estimated mean and 95% CI of AKI incidence in the patients with age ≤60 years and those with median or mean of time between symptom onset and hospitalization>7 days also determined. In addition, it was assessed the correlation between the incidence rates of AKI and the other complications (ARDS, ACI, secondary infection and sepsis/shock).
All meta-analyses and forest plots were performed in Microsoft Excel, following the step-by-step proposed by Neyeloff et al.14 The studies heterogeneity was assessed by Higgins inconsistency test (I2)15 and those with I2>50% were analyzed by random-effect model. The correlation tests were performed at software R, version 3.5.0. To perform the analyses, first, the data distribution was assessed (by the Shapiro–Wilk test) and, having found the absence of normality, Spearman correlation tests were applied. Statistically significant correlation was considered when the test p-value was<0.05.
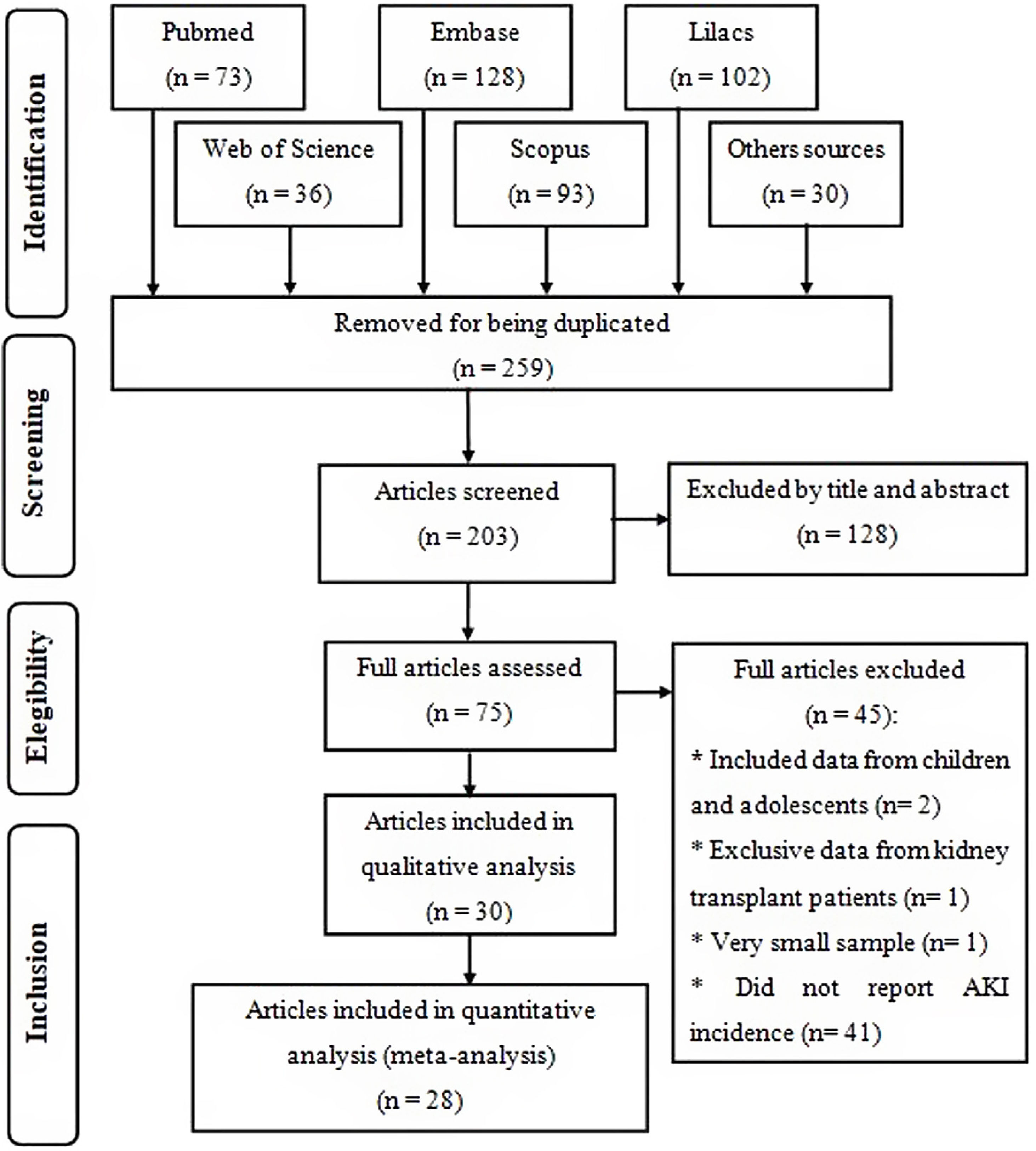
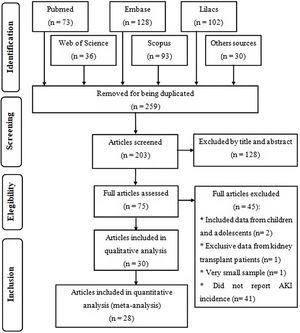
ResultsStudy descriptionInitially, 462 studies were found, from which 259 were removed for being duplicated. After reading the title and abstract of the remaining articles, 75 eligible publications were read in full, from which 30 were selected to compose this systematic review for meeting the previously established criteria, of which 28 were included in the meta-analysis (Fig. 1), where the two excluded studies were considered outliers and their exclusion from the quantitative analysis occurred to reduce heterogeneity between studies.
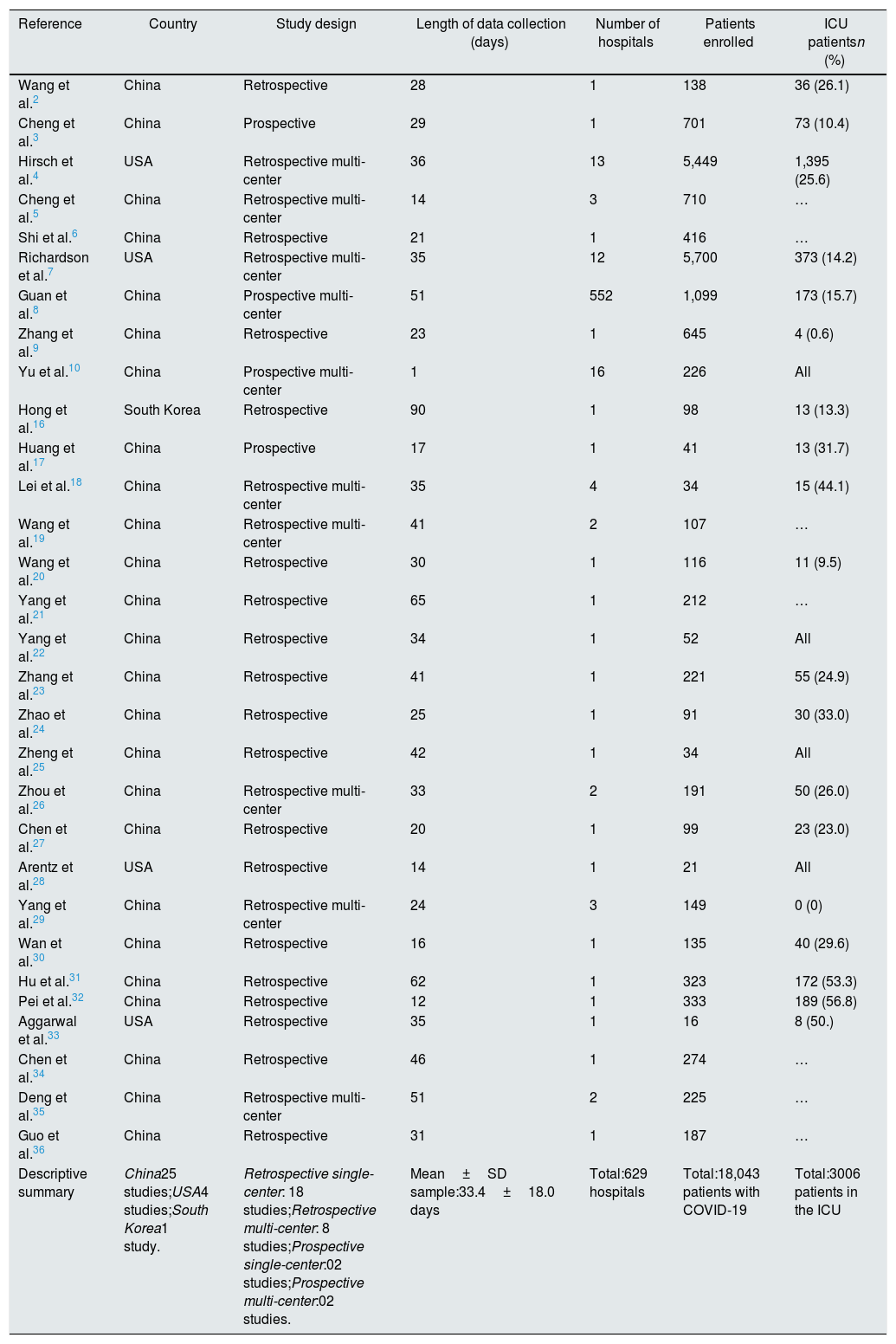
All articles were published in 2020 and together included 18,043 adult patients, with COVID-19 and that were hospitalized in 629 hospitals. The majority located in China and the length of data collection was between one and 90 days (Table 1).
Features and description of studies selected.
| Reference | Country | Study design | Length of data collection (days) | Number of hospitals | Patients enrolled | ICU patientsn (%) |
|---|---|---|---|---|---|---|
| Wang et al.2 | China | Retrospective | 28 | 1 | 138 | 36 (26.1) |
| Cheng et al.3 | China | Prospective | 29 | 1 | 701 | 73 (10.4) |
| Hirsch et al.4 | USA | Retrospective multi-center | 36 | 13 | 5,449 | 1,395 (25.6) |
| Cheng et al.5 | China | Retrospective multi-center | 14 | 3 | 710 | … |
| Shi et al.6 | China | Retrospective | 21 | 1 | 416 | … |
| Richardson et al.7 | USA | Retrospective multi-center | 35 | 12 | 5,700 | 373 (14.2) |
| Guan et al.8 | China | Prospective multi-center | 51 | 552 | 1,099 | 173 (15.7) |
| Zhang et al.9 | China | Retrospective | 23 | 1 | 645 | 4 (0.6) |
| Yu et al.10 | China | Prospective multi-center | 1 | 16 | 226 | All |
| Hong et al.16 | South Korea | Retrospective | 90 | 1 | 98 | 13 (13.3) |
| Huang et al.17 | China | Prospective | 17 | 1 | 41 | 13 (31.7) |
| Lei et al.18 | China | Retrospective multi-center | 35 | 4 | 34 | 15 (44.1) |
| Wang et al.19 | China | Retrospective multi-center | 41 | 2 | 107 | … |
| Wang et al.20 | China | Retrospective | 30 | 1 | 116 | 11 (9.5) |
| Yang et al.21 | China | Retrospective | 65 | 1 | 212 | … |
| Yang et al.22 | China | Retrospective | 34 | 1 | 52 | All |
| Zhang et al.23 | China | Retrospective | 41 | 1 | 221 | 55 (24.9) |
| Zhao et al.24 | China | Retrospective | 25 | 1 | 91 | 30 (33.0) |
| Zheng et al.25 | China | Retrospective | 42 | 1 | 34 | All |
| Zhou et al.26 | China | Retrospective multi-center | 33 | 2 | 191 | 50 (26.0) |
| Chen et al.27 | China | Retrospective | 20 | 1 | 99 | 23 (23.0) |
| Arentz et al.28 | USA | Retrospective | 14 | 1 | 21 | All |
| Yang et al.29 | China | Retrospective multi-center | 24 | 3 | 149 | 0 (0) |
| Wan et al.30 | China | Retrospective | 16 | 1 | 135 | 40 (29.6) |
| Hu et al.31 | China | Retrospective | 62 | 1 | 323 | 172 (53.3) |
| Pei et al.32 | China | Retrospective | 12 | 1 | 333 | 189 (56.8) |
| Aggarwal et al.33 | USA | Retrospective | 35 | 1 | 16 | 8 (50.) |
| Chen et al.34 | China | Retrospective | 46 | 1 | 274 | … |
| Deng et al.35 | China | Retrospective multi-center | 51 | 2 | 225 | … |
| Guo et al.36 | China | Retrospective | 31 | 1 | 187 | … |
| Descriptive summary | China25 studies;USA4 studies;South Korea1 study. | Retrospective single-center: 18 studies;Retrospective multi-center: 8 studies;Prospective single-center:02 studies;Prospective multi-center:02 studies. | Mean±SD sample:33.4±18.0 days | Total:629 hospitals | Total:18,043 patients with COVID-19 | Total:3006 patients in the ICU |
In eleven studies3,10,25,28,31,33,34 the median or mean age of enrolled patients was greater than 60 years, while the time between symptom onset and hospitalization was longer than 7 days in eight studies.3,5,6,22,25,31,32,34 The ARDS, ACI, secondary infection and sepsis/shock complication is reported by 22,2,6,8–10,16–20,22,23,25–28,30,31,33–36 19,2,6,10,16–19,21–26,28,30,31,33–35 1210,17–19,22,23,26–28,30,31,33 and 15 studies,2,8–10,16–19,23,26,27,30,31,34,35 respectively (S2 File).
AKI occurrenceFrom 30 studies included in this systematic review, only two20,29 reported that patients did not develop AKI and were not included in the meta-analysis. Among the studies that registered the occurrence of AKI, all used the KDIGO criteria for the AKI diagnosis and, by the crude analysis of the data, we verified that the overall incidence of the event was between 0.3%9 and 69%,33 while in the ICU the overall AKI incidence was between 2.9%8 and 53.2%.4 The highest rate of need of RRT was 22.7%.31 The crude mortality of patients with COVID-19 and AKI reached 100% in four studies19,26,34,35 (Table 2).
Data from AKI occurrence reported by studies selected.
| Reference | AKI definition | AKI incidence | Need of RRTN (%) | AKI mortality | ||
|---|---|---|---|---|---|---|
| OverallN (%) | ICUN (%) | Overall(%) | In RRTN (%) | |||
| Wang et al.2 | KDIGO | 5 (3.6) | 3 (8.3) | 2 (1.45) | … | … |
| Cheng et al.3 | KDIGO | 36 (5.1) | … | … | … | … |
| Hirsch et al.4 | KDIGO | 1993 (36.6) [Stage 1=927 (46.5); Stage 2=447 (22.4); Stage 3=619 (31.1)] | 1060 (53.2) [Stage 1=320 (34.5); Stage 2=244 (54.6); Stage 3=496 (80.1)] | 285 (14.3) | 694 (35) | … |
| Cheng et al.5 | KDIGO-sCr | 22 (3.2) [Stage 1=8 (1.3); Stage 2=6 (0.8); Stage 3=8 (1.1)] | … | … | … | … |
| Shi et al.6 | KDIGO | 8 (1.9) | … | 2 (0.5) | … | … |
| Richardson et al.7 | KDIGO-sCr | 523 (22.2) | … | 81 (3.2) | 347 (66.3) | … |
| Guan et al.8 | KDIGO | 6 (0.5) | 5 (2.9) | 9 (0.8) | … | … |
| Zhang et al.9 | … | 2 (0.3) | … | 0 (0) | … | … |
| Yu et al.10 | KDIGO | 57 (25.2) [Stage 1=23 (10.2); Stage 2=12 (5.3); Stage 3=22 (9.7)] | 57 (25.2) | 24 (10.6) | … | … |
| Hong et al.16 | KDIGO | 9 (9.2) | 8 (61.5) | 3 (3.1) | … | … |
| Huang et al.17 | KDIGO | 3 (7) | 3 (23) | 3 (7) | … | … |
| Lei et al.18 | KDIGO | 2 (5.9) | 2 (13.3) | 1 (2.9) | … | … |
| Wang et al.19 | KDIGO | 14 (13.1) | … | … | 14 (100) | … |
| Wang et al.20 | KDIGO | 0 (0) | … | … | … | … |
| Yang et al.21 | KDIGO | 28 (13.7) | … | … | 10 (35.7) | … |
| Yang et al.22 | KDIGO-sCr | 15 (29) | 15 (29) | 9 (17) | 12 (37.5) | 8 (25) |
| Zhang et al.23 | … | 10(4.5) | 8 (14.5) | 5 (2.3) | … | … |
| Zhao et al.24 | elevated sCr and uric acid levels | 5 (5.5) | 5 (16.7) | 3 (3.3) | … | … |
| Zheng et al.25 | KDIGO | 7 (20.6) | 7 (20.6) | 5 (14.7) | … | … |
| Zhou et al.26 | KDIGO | 28 (15) | … | 10 (5) | 27 (96.4) | 10 (100) |
| Chen et al.27 | … | 3 (3) | … | 9 (9) | … | … |
| Arentz et al.28 | KDIGO-sCr | 4 (19.1) | … | … | … | … |
| Yang et al.29 | … | 0 (0) | … | … | … | … |
| Wan et al.30 | … | 5 (3.7) | 1 (2.5) | 5 (3.7) | … | … |
| Hu et al.31 | … | 17 (5.3) | 15 (8.7) | 72 (22.3) | … | … |
| Pei et al.32 | KDIGO and KDIGO-exp | KDIGO: 22 (6.6) [Stage 1=4 (18.2); Stage 2=7 (31.8); Stage 3=11 (50)];KDIGO-exp: 35 (10.5) [Stage 1=16 (45.7); Stage 2=8 (22.9); Stage 3=11 (31.4)] | 30 (15.9) | … | KDIGO: 20 (57.1) [Stage 1=4 (25); Stage 2=6 (75); Stage 3=10 (90.9)]; KDIGO-exp: 19 (86.4) [Stage 1=3 (75); Stage 2=6 (85.7); Stage 3=10 (90.9) | … |
| Aggarwal et al.33 | … | 11 (69) | 3 (38) | … | … | … |
| Chen et al.34 | KDIGO | 29 (11) | … | 3 (1) | 28 (96.6) | 3 (100) |
| Deng et al.35 | … | 20 (8.9) | … | … | 20 (100) | … |
| Guo et al.36 | … | 18 (14.6) | … | … | … | … |
Footnote: KDIGO-sCr: Only the serum creatinine of KDIGO criteria; KDIGO-exp: KDIGO with expanded criteria; Legend: AKI: acute kidney injury; ICU: intensive care unit; RRT: renal replacement therapy; KDIGO: Kidney Disease Global Improving Outcomes; sCr: serum creatinine.
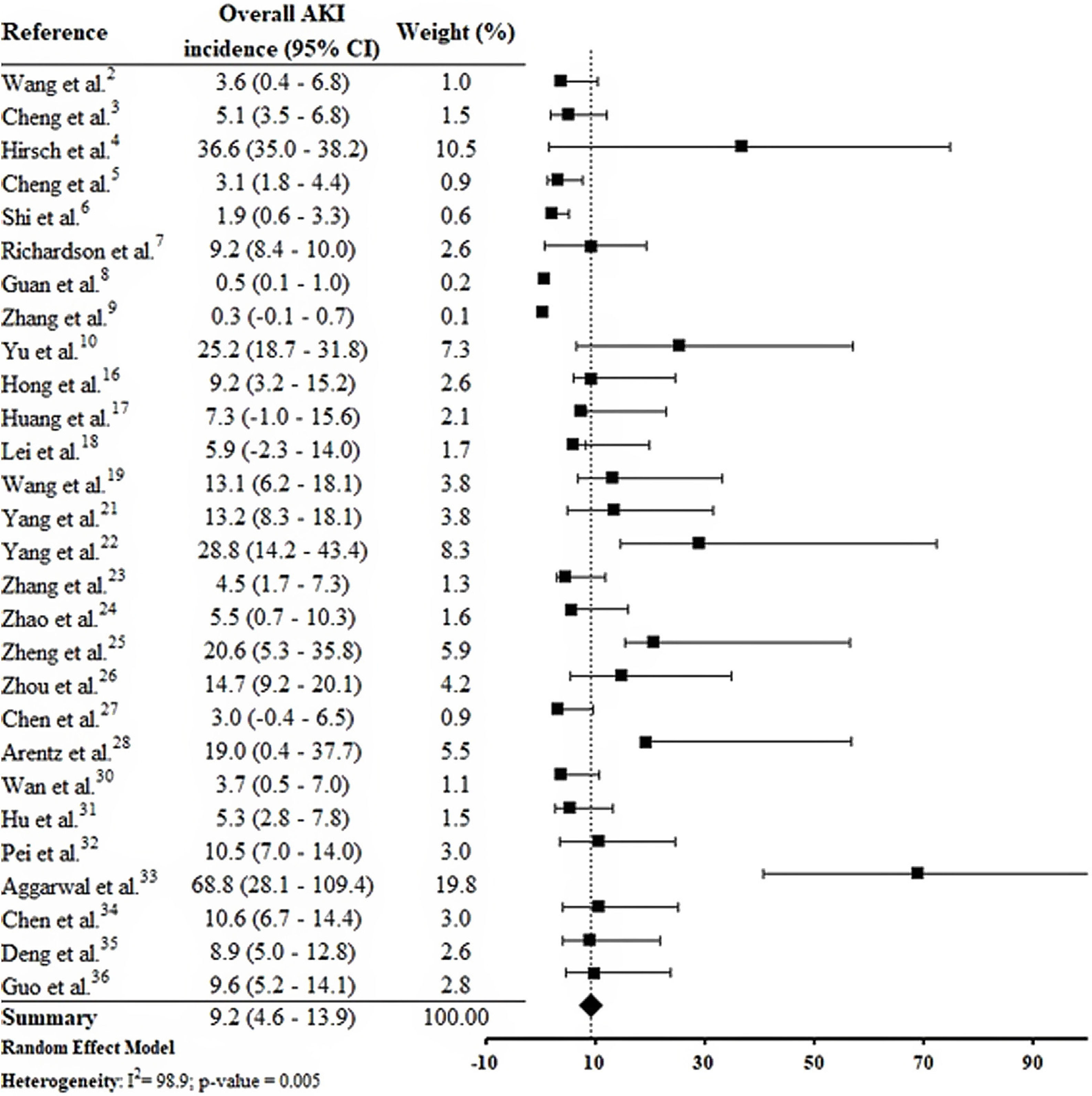
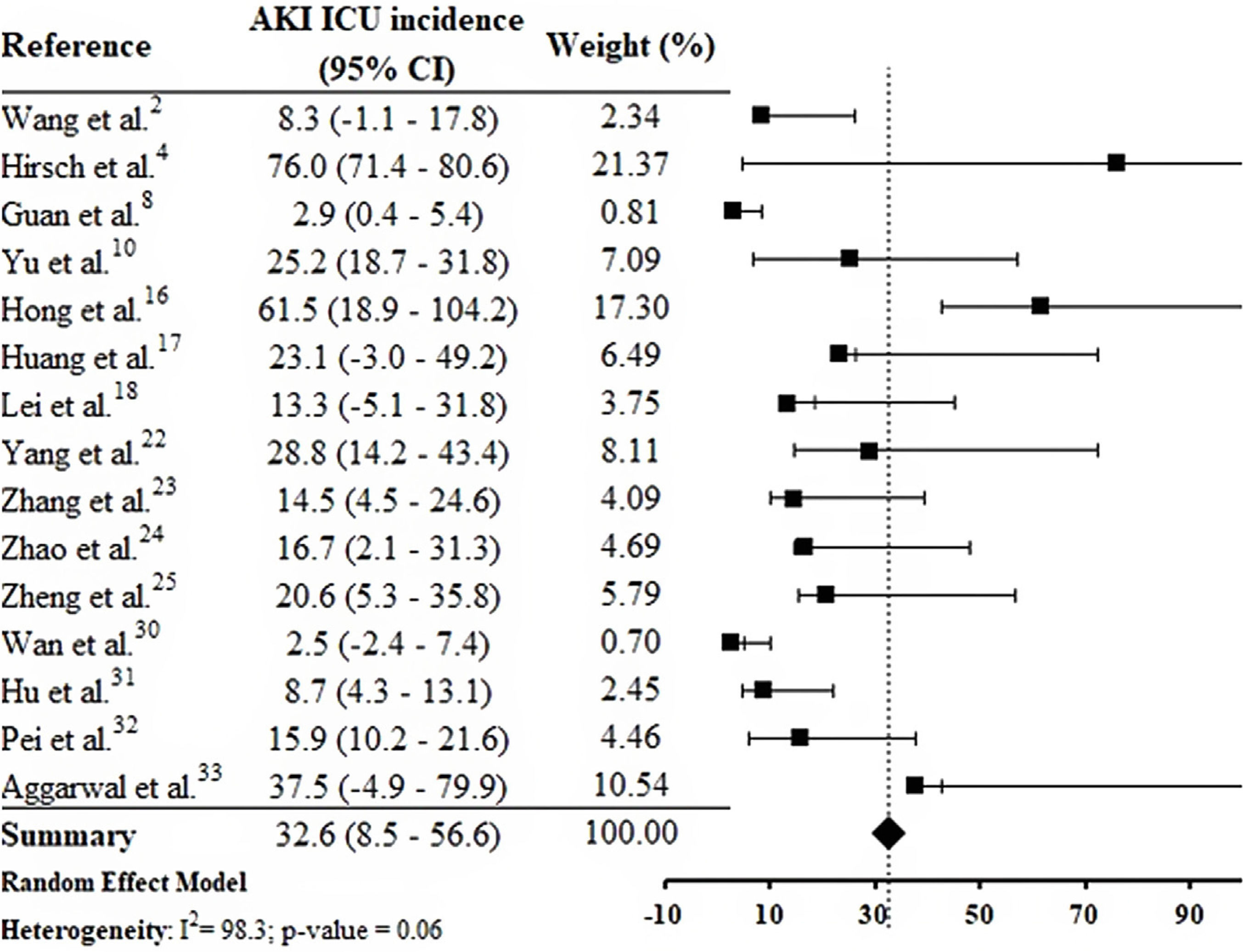
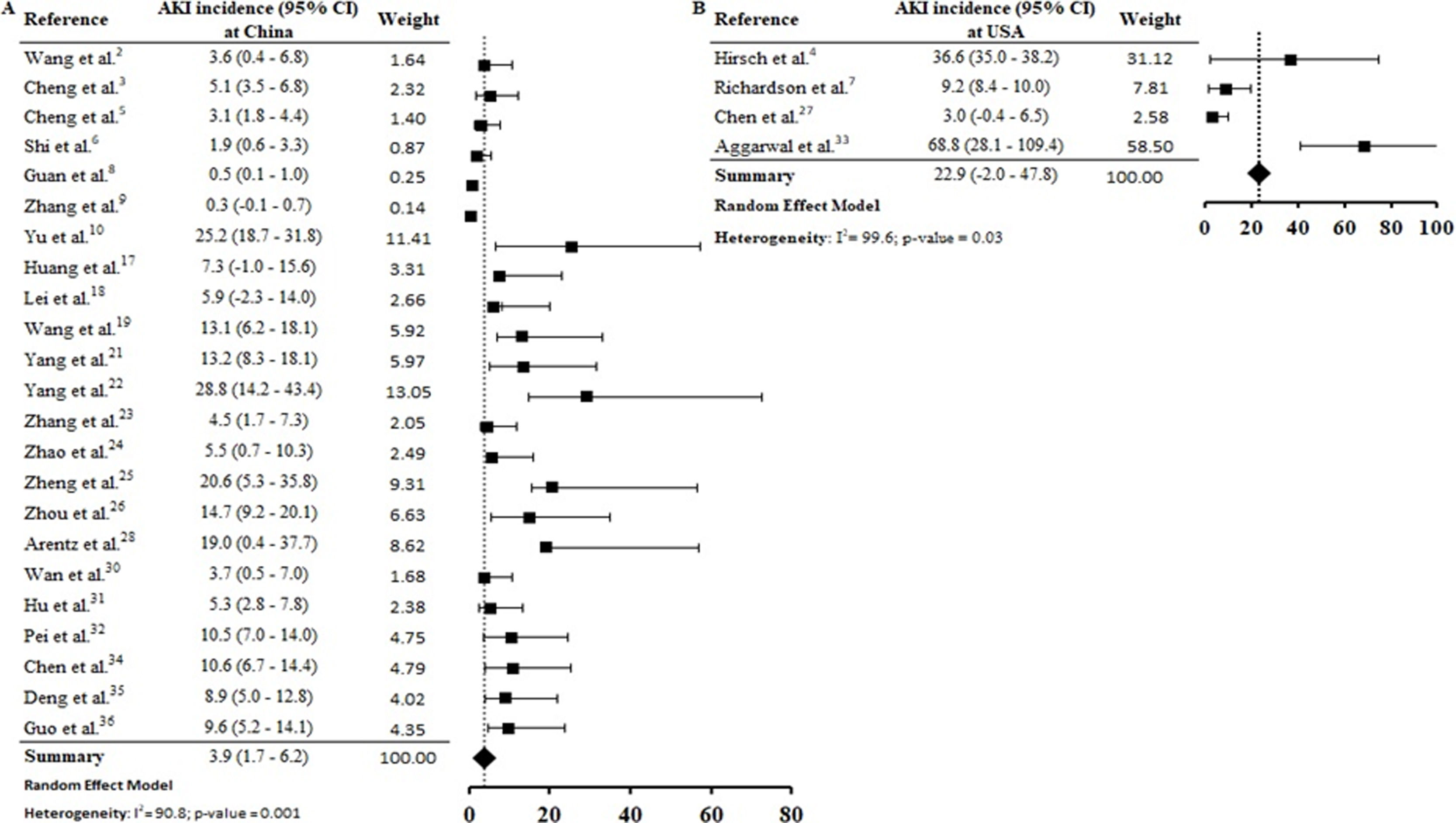
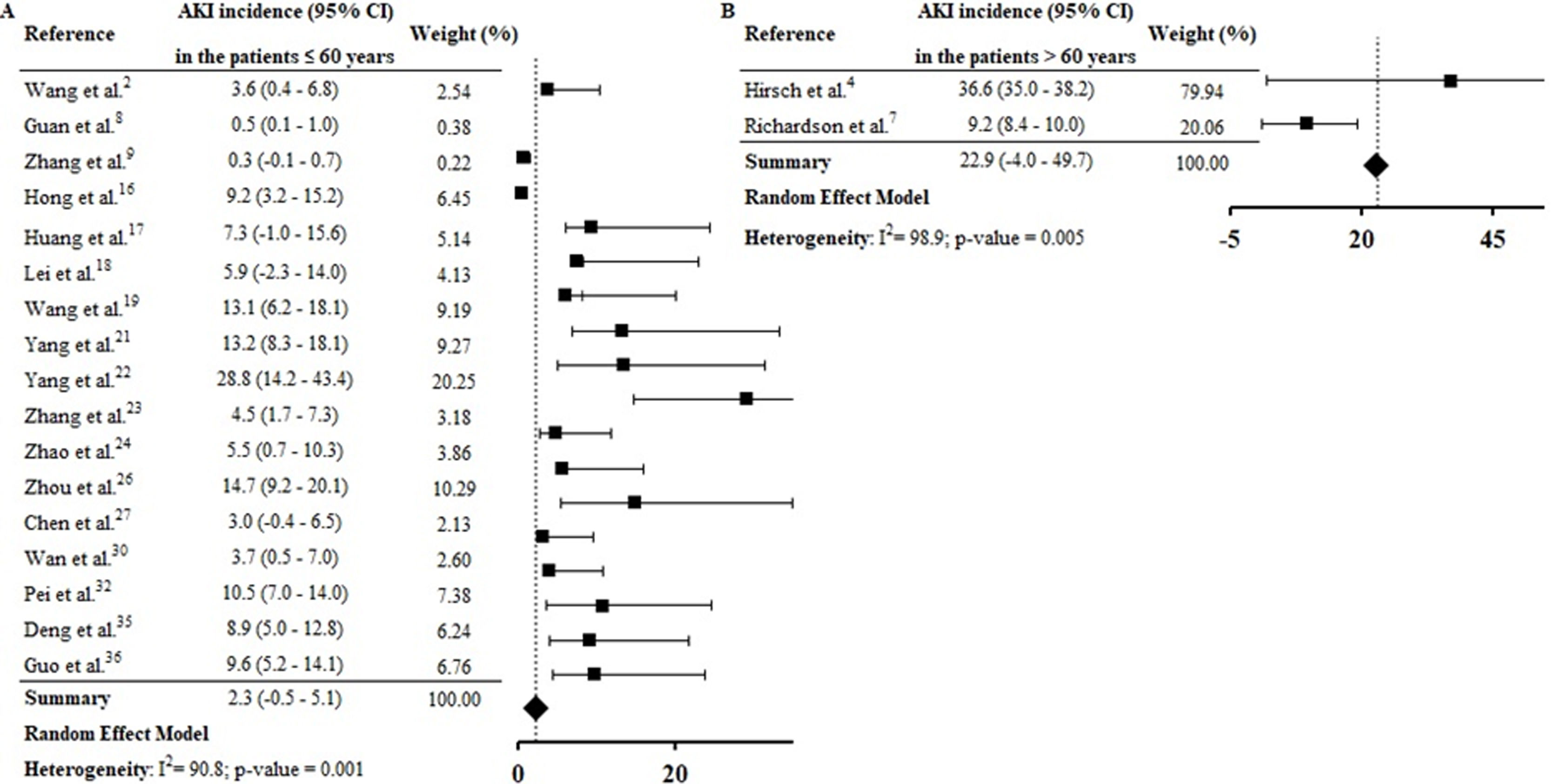
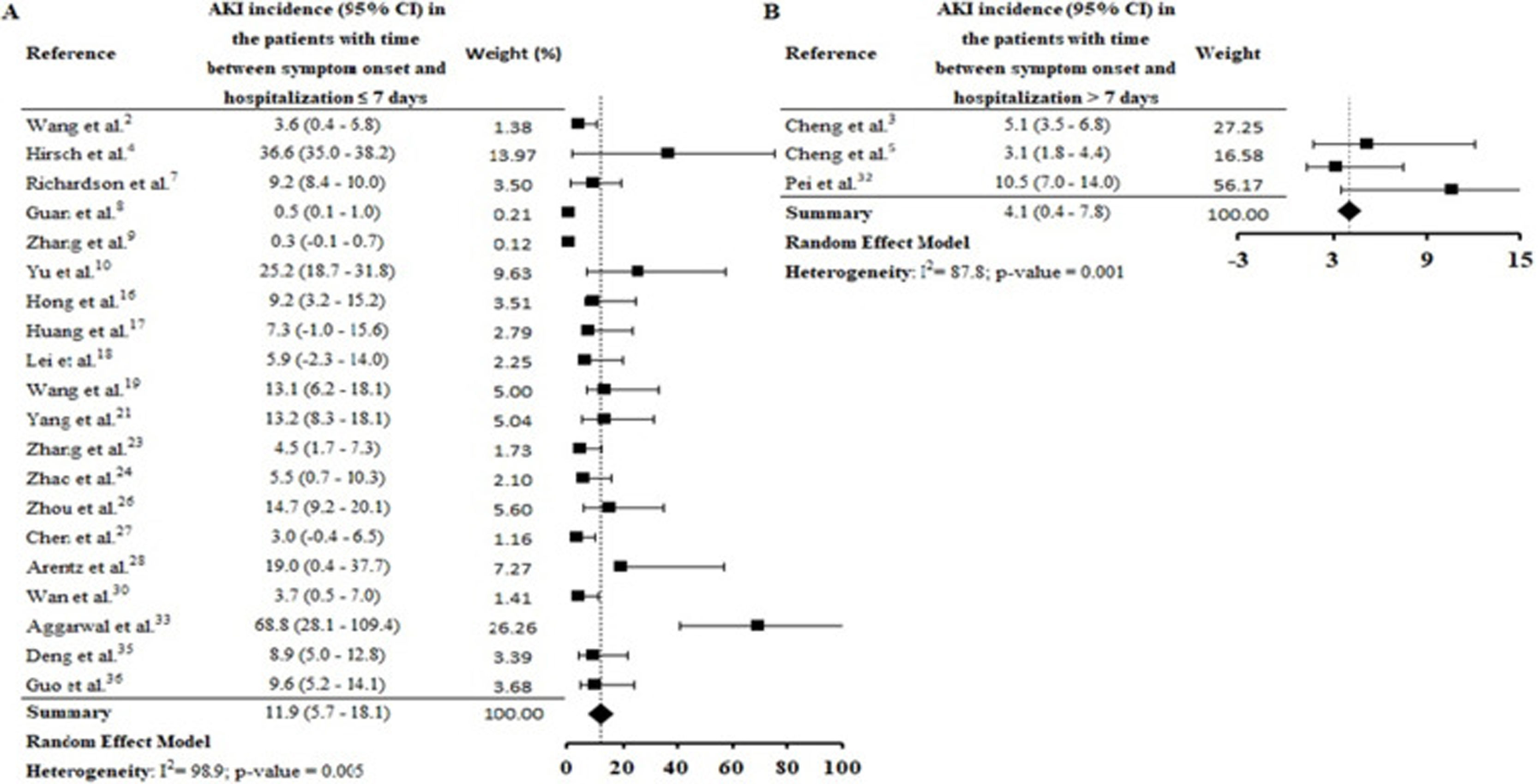
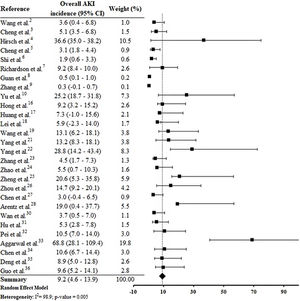
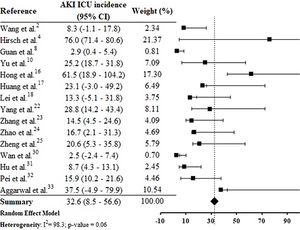
By the meta-analysis of the assessed studies, the estimated mean and 95% CI of AKI incidence overall was 9.2% (CI 4.6–13.9%) (Fig. 2) while at the ICU was 32.6% (CI 8.5–56.6%) (Fig. 3), both with a high heterogeneity index (I2=98.4% and 98.3%, respectively). According to the subgroup analysis, we verified that AKI incidence at USA, elderly patients and those with early hospitalization was 22.9% (CI −2.0–47.8%; I2=90.8%) (S3 File), 22.9% (CI −4.0–49.7%; I2=90.8%) (S4 File) and 11.9% (CI 5.7–18.1%; I2=99.9%) (S5 File), respectively.
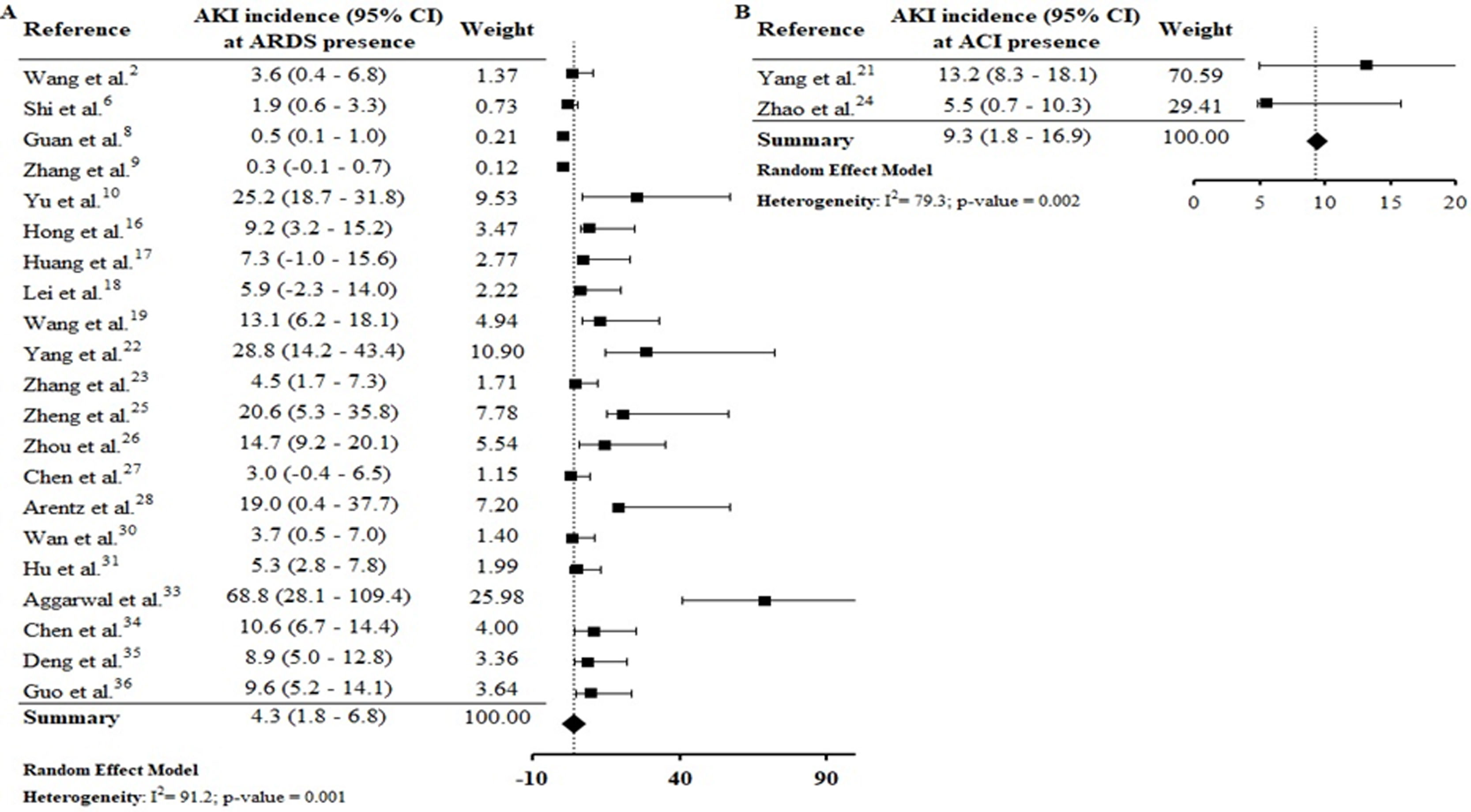
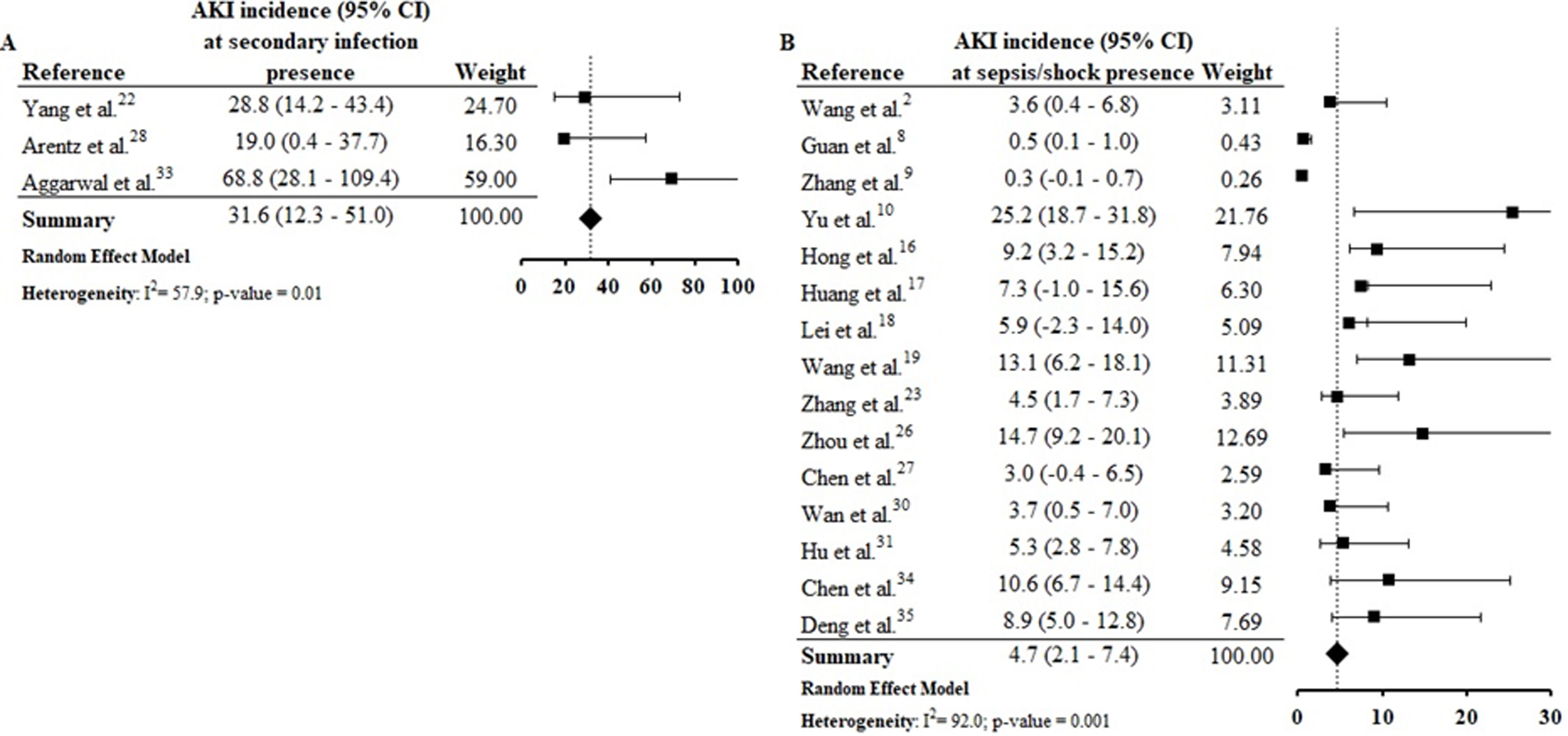
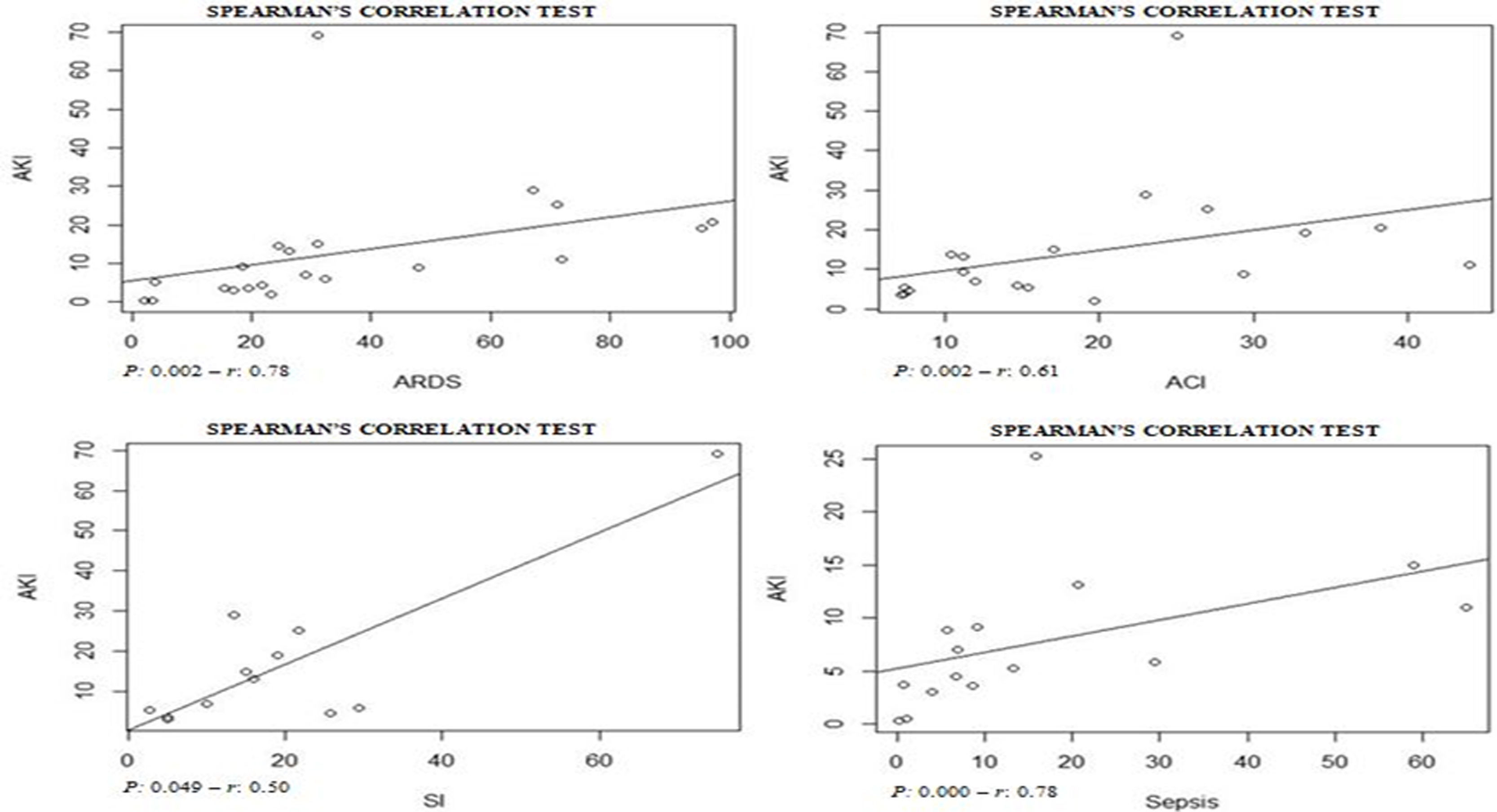
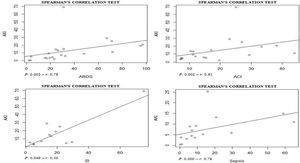
Assessing the AKI incidence in the presence of other complications, we verified that in patients with ARDS and ACI the AKI incidence was 4.3% (CI 1.8–6.8%; I2=91.2%) and 9.3% (CI 1.8–16.9%; I2=79.3%) (S6 File), respectively. In addition, the AKI incidence in patients with secondary infection was 31.6% (CI 12.3–51.0%; I2=57.9%), while that in patients with sepsis/shock the AKI incidence was 4.7% (CI 2.1–7.4%; I2=92%) (S7 File). By Spearman correlation test, we identified that ARDS and AKI occurrence present a direct and statistically significant correlation (S8 File).
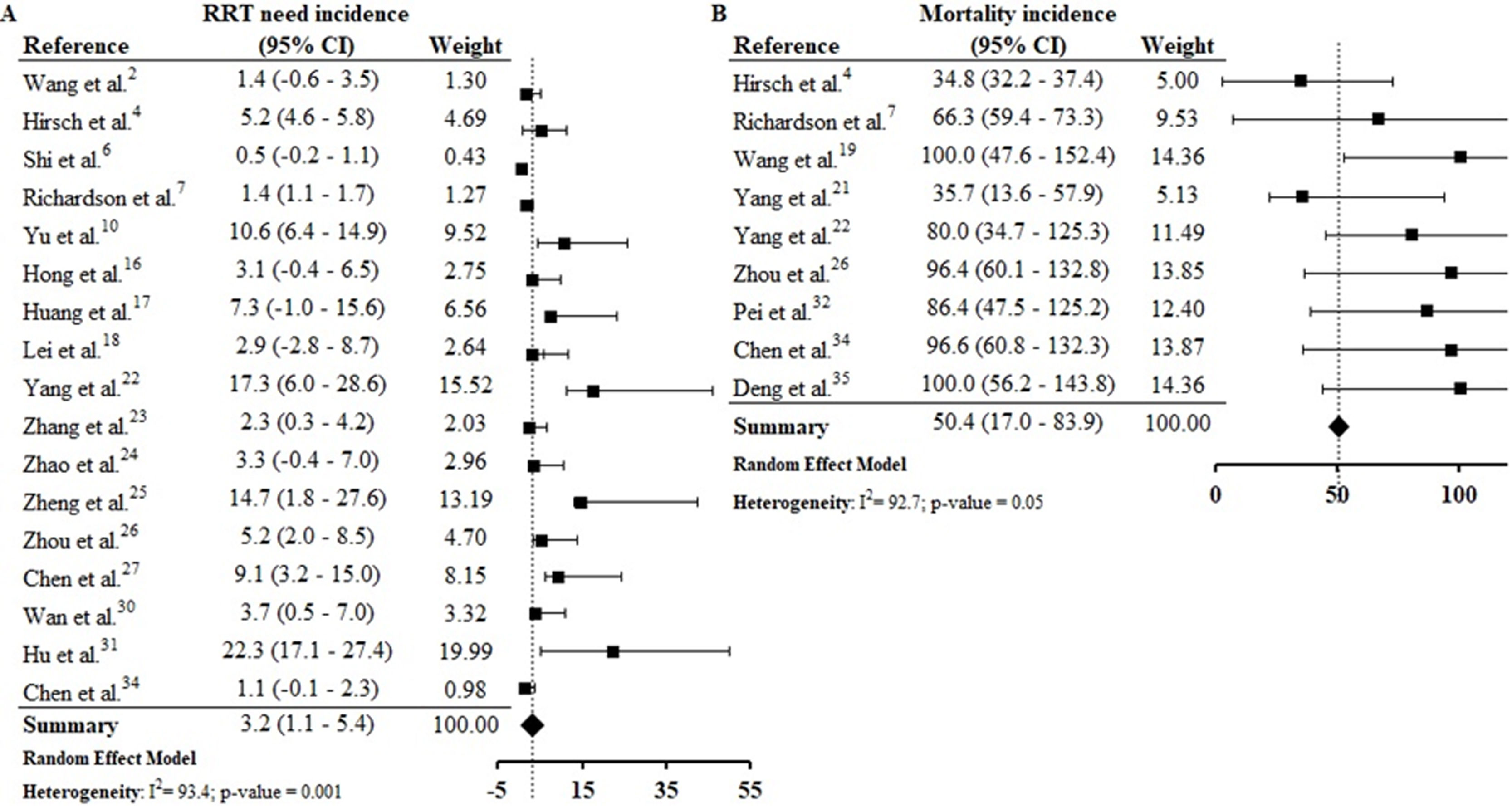
From 30 studies included in this systematic review 172,4,6,7,10,16–18,22–27,30,31,34 reported data from RRT need and, by meta-analysis we found that 3.2% (CI 1.1–5.4%) of patients with COVID-19 presented RRT need. The assessment of combined mortality of patients with COVID-19 AKI-associated made it possible to determine that the mean proportion of death was 50% (CI 17.0–83.9%) (S9 File).
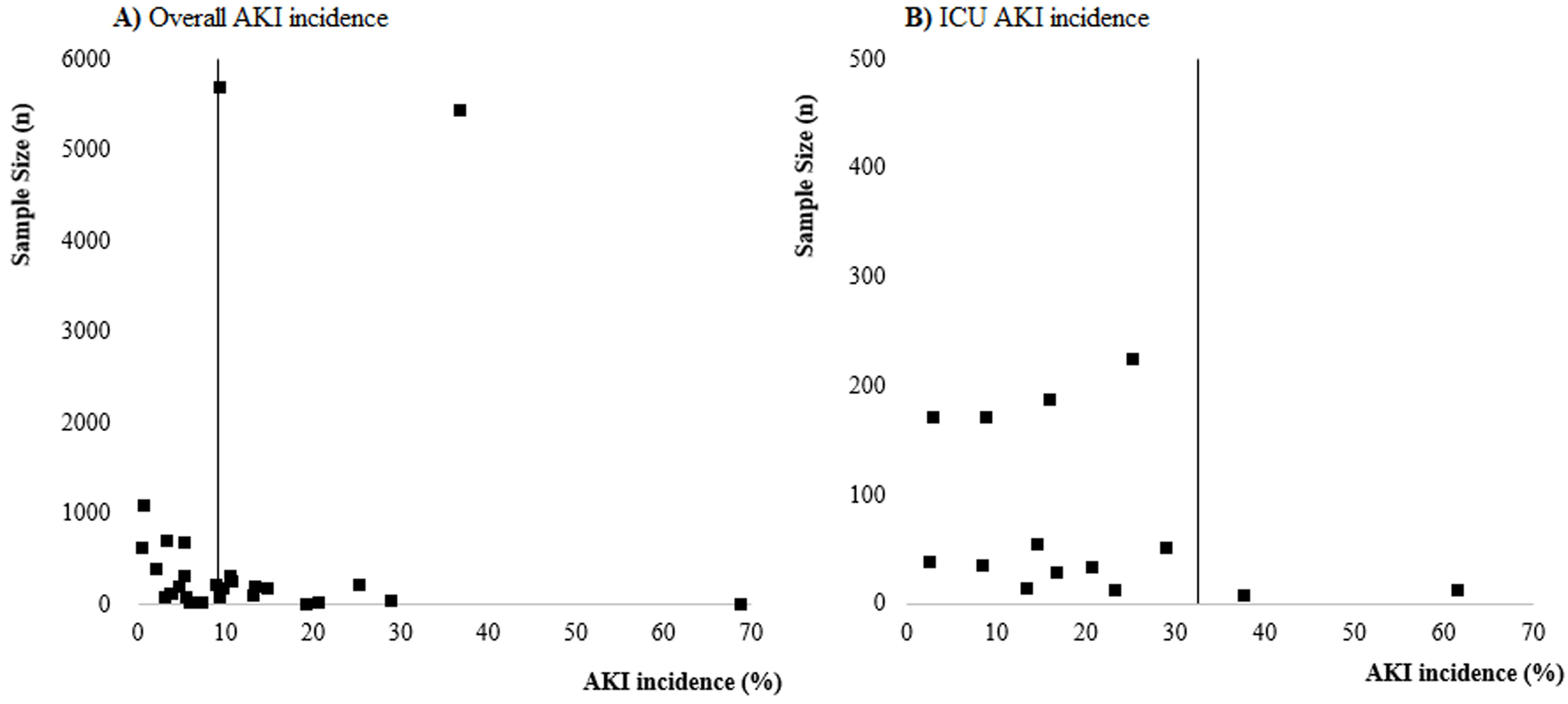
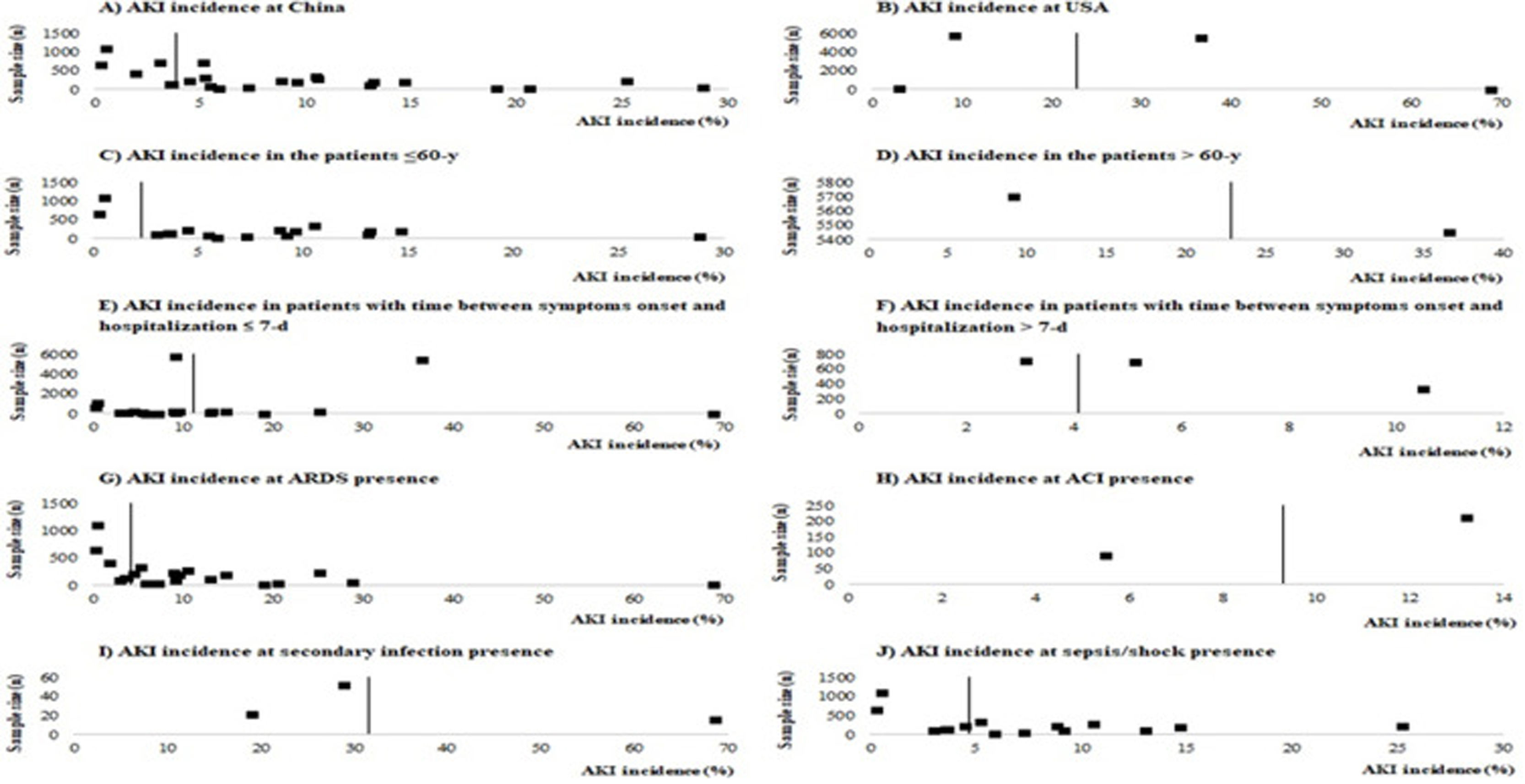
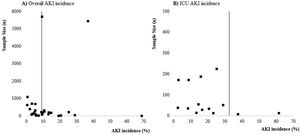
Risk of biasNo study presented high risk of bias and 23 studies2,8–10,18–26,29,30,32,34–36 presented low risk of bias. The main methodological limitations between the studies were related to the lack of information if they identified and dealt with confounding factors (S10 File). The funnel plots (S11 and S12 Files) present the function of sample size of studies with AKI incidence, suggesting a possible risk of publication bias.
DiscussionThis systematic review was conducted from a comprehensive search in the main biomedical databases of the world and included 30 studies published until the third week of Jun 2020, which together report data from more than 18,000 patients (table 1). By the meta-analysis from 28 studies, it was verified that 9.2% (CI 4.6–13.9%) of adult patients hospitalized with COVID-19 presented AKI (Fig. 2). Among patients that required admission to ICU the AKI incidence was substantially higher (32.6%, CI 8.5–56.6%) (Fig. 3).
We verified that the meta-analysis of AKI overall and ICU incidence presented high heterogeneity and, possible causes for this may be related to recognized differences among the studies, especially, with regard to design, number of included participants and follow-up time. The number of enrolled patients varied between 16 patients in a single-center retrospective study conducted in the USA33 and 5700 enrolled in a retrospective multi-center study also conducted in the USA.7
In the research of Hirsch et al.,4 53.2% of patients with AKI were in the ICU. Yu et al.10 assessed 226 critically patients with COVID-19 in 19 ICUs from Wuhan, China, and the AKI incidence was 25.2%. In one study carried out in South Korea, AKI incidence in the ICU was 61.5%.16
From what is known so far, among the mechanisms involved in the AKI occurrence in patients with COVID-19 is the triggering of the cytokine release syndrome (CRS), also known as “cytokine storm”, with consequent endothelial damage, mitochondrial dysfunction, hypovolemia, and hypercoagulability.37,38 Moreover, the direct viral damage, mediated by co-expression and activity of angiotensin-converting enzyme 2 (ACE2) and cellular transmembrane serine proteases (TMPRSSs), can cause kidney damage cells and apoptosis, especially, in podocytes and proximal straight tubule cells.39,40
These multiple pathophysiological depletions can be observed in the clinical practice by identifying some renal abnormalities.3,7,21,32,37 By data analysis of 333 patients, Pei et al.32 observed that 75.4% presented renal abnormalities, being proteinuria and hematuria the most frequent. These are also the most frequent abnormalities observed in the research of Cheng et al.3 Furthermore, data from other studies in which the AKI occurrence were reported also showed that the patients had elevated levels of serum creatinine and BUN.4,8,16,17,21,26,27,30,31
We performed subgroup analyses to minimize the high heterogeneity observed in the global analysis (with all studies included) Even so, the high heterogeneity remained in the subgroup analysis. Even grouping the studies in which the patients had similar characteristics, the difference in the number of patients enrolled, possibly, was the main cause of maintaining high heterogeneity.
According to geographic location of these COVID-19 cases, the AKI estimate incidence was vastly higher at USA (22.9% [CI −2.0–47.8%]) than China (3.9 [CI 1.7–6.2%]) (S3 File). This expressive difference in the AKI incidence between the two countries, could be explained at least in part, by the fact that Occidental populations have a more pronounced expression of the receptor ACE2 in podocytes and proximal straight tubule cells.39 Hirsch et al.4 identified higher AKI incidence among black (20.8%) and white (41%) patients than in Asians (8.1%). However, other epidemiological and experimental studies, including data with patients from different races are needed to confirm this preliminary evidence.
In this systematic review, high AKI incidence was also found among elderly (S4 File) and patients with early hospitalization (S5 File). Hirsch et al.4 identified that advanced age is an independent risk factor for AKI, and the highest incidence of this complication on COVID-19 patients was verified in the first days of hospitalization. Yang et al.21 highlighted that elderly patients had a worse prognosis after COVID-19 infection and 14% of patients with hospitalization≤7 days had essential organ injury, including AKI.
Thereby, AKI incidence seems to be linked to the shorter onset time of COVID-19, given by time between symptoms onset and hospitalization. Pan et al.39 report that co-expression of the ACE2 and TMPRSS genes in kidney cells were similar to that seen in the lung and other organs. In addition, the authors highlight that the time between detection of COVID-19 in blood and AKI occurrence was around one week.
Based on the findings of this study and in the literature, it is reasonable to believe that the AKI occurrence in adult patients hospitalized with COVID-19 is as early as acute respiratory failure, and the health team must be attentive to the predictive signs of AKI occurrence, as described in KDIGO (Kidney Disease: Improving Global Outcomes) guidelines for care guidance aiming the early diagnosis and treatment.3,32,37
AKI incidence among patients with ARDS (4.3% [CI 1.8–6.8%]) was lower than in those with ACI (9.3% [CI 1.8–16.9%] (S6 File). By the correlations test, ARDS and ACI showed a direct and statistically significant correlation of greater strength with the AKI incidence (S8 File). Shi et al.6 verified that ACI, with an incidence of 19.7%, was significantly associated with AKI (8.5%) and ARDS (58.5%) incidences. In a study performed by Guo et al.36 with patients from Wuhan, China, the authors observed that myocardial injury was significantly associated with worse outcome. In addition, the AKI and ARDS incidences were higher in patients with elevated troponin T levels, compared to those with normal levels.
In a robust study from the USA, the need for mechanical ventilation (one of the ARDS consequences), as well as cardiovascular disease (including heart failure) were significant risk factors for AKI occurrence.4 In the COVID-19 infection, injury in the lung-kidney-heart triad is associated with direct viral damage due to high local expression of ACE2 receptor, resulting in organ crosstalk (as alveolar damage, renal compartment syndrome, cardiomyopathy, cardiorenal syndrome and tubular toxicity) and systemic effects.
AKI estimate incidence in patients with secondary infection was 31.6% (CI 12.3–51.0%) and among those sepsis/shock was 4.7% (CI 2.1–7.4%) (S7 File). In the correlation tests, the incidence of these complications was also directly proportional to the AKI incidence (S9 File). Among selected studies, the rates of secondary infection and sepsis/shock were between 2.8%30 and 75%33 and between 0.3%9 and 65%,34 respectively. These complications result in hemodynamic instability, contributing to the increased need for RRT and the mortality rate among patients.37–40
We emphasize that the studies included in this review present data collected at the beginning of the pandemic, when there were still many uncertainties about the pathophysiological mechanisms involved in the occurrence of AKI in patients with COVID-19. Thus, the possible difficulty in identifying the occurrence of AKI in critically ill patients with sepsis/shock before they died may, at least in part, justify the low incidence of AKI in patients with COVID-19 and sepsis/shock, compared to those with secondary infection. Therefore, we encourage the conduction of other review studies like this one, to identify possible changes in the current epidemiological profile of AKI in patients with COVID-19 compared to what we present in this review, which brings data from studies conducted at the beginning of the pandemic.
The estimated proportion of RRT need was 3.2% (CI 1.1–5.4%) and the estimated incidence of mortality was 50.4% (CI 17.0–83.9%) (S12). Continuous RRT (CRRT) was the predominant dialysis technique reported by studies. Hu et al.31 reports that 22.3% of patients studied required CRRT and, among those that required ICU admission the CRRT rate was 26.7%. The rate of death in AKI was between 35.7%21 and 100%,19,35 and the mortality rate was higher among patients who required CRRT.26,34
Recommendations for practice and for researchViral tropism of SARS-CoV-2 is believed to be among the main causes of direct kidney damage. Thus, early recognition of AKI episodes in patients with COVID-19 is even more essential to avoid the rapid progression of the disease and the negative outcome. It is recommended that patients be stratified for AKI risk based on the presence of comorbidities, demographic data, and clinical conditions, establishing the monitoring of renal function from hospital admission and continuously during hospitalization.
Considering the lack of specific treatment for AKI in COVID-19, instituting intensive care support is among the most favorable existing options, especially among patients with severe COVID-19.4,37,38,40 Based on clinical experience, it is suggested that instituting extracorporeal therapies may assist in removing cytokines as well as can be usefulness for minimizing the organ crosstalk and systemic effects.37,38 However, considering the high demand, the lack of structure and technological resources (dialysis machines) and human resources (specialized team in nephrology) can be a reality experienced by many countries. Thus, promoting prevention strategies and early identification of AKI, in order to prevent this complication from progressing, is the gold standard of care. To improve the quality of kidney care, the 25th Acute Disease Quality Initiative (ADQI) consensus report presents recommendations for management of AKI associated with COVID-19.41
A better understanding of the mechanisms involved in the occurrence of AKI in patients with COVID-19 is still needed. It is recommended that patients with AKI associated to COVID-19 be evaluated for the course and time of AKI. It is necessary to develop robust scientific studies with high methodological rigor, to verify whether the risk factors for AKI in COVID-19 differ from those already known, as well as to know the factors associated with the recovery of renal function in the short and long term. In addition, knowing the epidemiological burden of AKI in patients who, in addition to COVID-19, already have previous conditions of vulnerability (CKD, cancer, other infectious diseases, transplants, surgeries and others) is equally important.
LimitationsThis systematic review and meta-analysis have some limitations. First, the most selected studies were from China and with considerable risk of methodological biases once they come from retrospective case series or observational analysis. In addition, most studies report data from a restricted sample of patients. The funnel plots (S11 and S12 Files) suggest a possible publication bias and bias related, especially to the fact that most studies were not designed to assess AKI incidence and, as consequences, the definition of AKI was unclear in most of these studies. Although KDIGO was reported as being used in several studies, information regarding rate and definition of baseline serum creatinine, urine output criteria or period of observation to define AKI were not described. In the analyses of the studies included in this systematic review we focused our efforts on identifying the incidence of AKI and, because of that, we did not collect data on risk factors for AKI occurrence. Thus, we recommend that future studies include such an analysis to better understand the epidemiology of AKI in COVID-19.
ConclusionsThe occurrence of AKI is frequent among adult patients hospitalized with COVID-19, and affects on average, up to 13.9% of these patients. It is believed that AKI occurs early and in parallel with lung injury. The elderly population, with acute cardiac injury and secondary infection, is among the group at highest risk for AKI.
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors declare that there is no conflict of interest.