Nuclear receptor binding protein 1 (NRBP1) and ATP-binding cassette subfamily G member 2 (ABCG2) was the gout risk gene and high-capacity urate exporter respectively. However, the relationship between NRBP1 and ABCG2 and the underlying molecular mechanism contributing to these associations are unknown.
MethodsFirstly, the efficiency of the overexpression and knockdown of NRBP1 was confirmed by western blot. Next, the effect of NRBP1 overexpression and knockdown on the expression of ABCG2, organic anion transporter 1 (OAT1), glucose transporter 9 (GLUT9) and urate transporter 1 (URAT1) was detected by qRT-PCR and western blot. At the same time, the cellular location of ABCG2 and its expression after NRBP1 overexpression and knockdown was tested by immunofluorescence (IF) staining. Then, the mechanism of NRBP1 modulates ABCG2 expression was evaluated by western blot with or without the β-catenin inhibitor (21H7).
ResultsThe lentivirus system was used to generate stable NRBP1 overexpression, while the plasmids carrying a NRBP1 siRNA was generated to knockdown NRBP1 expression in HK-2 cells. Meanwhile, the overexpression of NRBP1 significantly decreased the mRNAs and proteins expression of GLUT9 and URAT1, while the knockdown of NRBP1 increased the mRNAs and proteins expression of ABCG2 significantly. In addition, the NRBP1 modulates the expression of ABCG2 was by ctivating the Wnt/β-catenin pathway in HK-2 cells according to the IF and western blot results.
ConclusionTaken together, our study demonstrated that NRBP1 inhibition played an essential role in attenuating hyperuricemia and gout by upregulation of ABCG2 via Wnt/β-catenin signaling pathway in HK-2 cells.
La proteína de unión al receptor nuclear 1 (NRBP1) y el miembro G de la subclase ATP binding Box 2 (ABCG2) son los genes de riesgo de gota y los genes de salida de urato de alto rendimiento, respectivamente. Sin embargo, se desconoce la relación entre NRBP1 y ABCG2, y los posibles mecanismos moleculares que conducen a estas asociaciones.
MétodosEn primer lugar, la sobreexpresión y el knockout de NRBP1 fueron confirmados por Western-blot. Los efectos de la sobreexpresión y knockout de NRBP1 en la expresión de ABCG2, transportador de aniones orgánicos 1 (OAT1), transportador de glucosa 9 (GLUT9) y transportador de ácido úrico 1 (URAT1) fueron detectados por qRT-PCR y Western-blot. Mientras tanto, la localización y expresión de ABCG2 después de la sobreexpresión y knockout de NRBP1 fueron detectadas por inmunofluorescencia (IF). Luego, el efecto regulador de NRBP1 sobre la expresión de ABCG2 fue estudiado por Western-blot y comparado con el inhibidor de la β-catenina (21H7).
ResultadosEl sistema lentiviral indujo una sobreexpresión estable de NRBP1, mientras que el plásmido portador de SiRNA NRBP1 inhibió la expresión de NRBP1 en las células HK-2. Mientras tanto, la sobreexpresión de NRBP1 redujo significativamente la expresión de ARNm y proteínas de GLUT9 y URAT1, mientras que el knockout de NRBP1 aumentó significativamente la expresión de ARNm y proteínas de ABCG2. Además, de acuerdo con los resultados de IF y Western-blot, NRBP1 regula la expresión de ABCG2 activando la vía Wnt/β-catenina en las células HK-2.
ConclusiónLa inhibición del NRBP1 aumenta la regulación de ABCG2 a través de la vía de señalización Wnt/β-catenina, que desempeña un papel importante en la reducción de la hiperuricemia y la gota.
Gout is a serious health issue with a prevalence of 1.14% in adults in China,1 and is an independent risk factor for heart failure and metabolic syndrome.2 It is associated with many conditions that affect longevity, such as hypertension, diabetes mellitus, metabolic syndrome, and renal and cardiovascular disease.3–6 Hyperuricemia is considered to be a precursor of gout as the deposition of urate crystals in the joints and soft tissue results in an acute inflammatory response and tophi, respectively.7,8 In recent years, the trend in the prevalence of gout and hyperuricemia have been observed increased in epidemiological studies,9,10 and the diseases have become public health problems that need to be solved quickly.
Generally, hyperuricemia has been classified into urate ‘overproduction type’ and/or ‘underexcretion type’ based solely on renal urate excretion, without considering an extra-renal pathway.11 Uric acid is principally derived from purine metabolism.12 And hyperuricemia was caused by metabolism abnormalities of serum uric acid.13 In humans, kidney plays a pivotal role in urate handling, the mechanism is to maintain the balance between secretory and efficient reabsorption.14 The reduction of glomerular filtration, increased tubular resorption, and decreased tubular secretion of uric acid all induced the impairment of renal excretion of uric acid.15 The excretion of uric acid in the kidney is assisted by uric acid transporters, which have uric acid reabsorption transporters and uric acid secretion transporters. The renal proximal nephron plays a major role in the reabsorption of glucose from the urine using a combination of sodium-coupled hexose transporters, urate transporter 1 (URAT1, SLC22A12) and glucose transporter 9 (GLUT9, SLC2A9).16 At the same time, the transporters of ATP-binding cassette subfamily G member 2 (ABCG2) and organic anion transporter 1 (OAT1, SLC22A6) play major roles in the uric acid secretion.17,18
Dozens of loci associated with gout were identified by genome-wide association studies, and nuclear receptor binding protein 1 (NRBP1) is one of the risk genes. At the same time, it has been confirmed that the expression of NRBP1 was elevated in human peripheral blood mononuclear cells from gout patients.1 Previous studies suggested that NRBP1 can inhibit the proliferation of tumor cells by activating Wnt/β-catenin signaling pathways.19,20 Several studies have also found that the activation of Wnt/β-catenin signaling pathway can lead to the expression of uric acid transporter ABCG2 decrease.21,22 ABCG2, as the most important uric acid transporter, is mainly distributed in the proximal renal tubule of the kidney, and its decreased expression is the pathophysiological basis for the occurrence of hyperuricemia and gout. Therefore, we hypothesized that NRBP1 could induce the expression of uric acid transporters ABCG2 decrease in the renal proximal nephron by activating the Wnt/β-catenin signaling pathway, to further induce the hyperuricemia.
In this study, we used the overexpression and knockdown of NRBP1 in the HK-2 cells to evaluate its effect on the uric acid transporter. In addition, the effects of blocking Wnt/β-catenin signaling pathways on NRBP1 mediated ABCG2 expression were also investigated. According to our observations, the overexpression of NRBP1 induced the expression of ABCG2, which promotes uric acid reabsorption and inhibits uric acid secretion. And the inhibition of NRBP1 on the expression of ABCG2 was by activating the Wnt/β-catenin pathway in HK-2 cells. This finding will help pinpoint the causes of hyperuricemia more accurately and provide a more effective therapeutic strategy for hyperuricemia and gout, leading to a good model of personalized medicine for common diseases.
Materials and methodsCells cultureHuman renal proximal tubular epithelial cells (HK-2 cells) were purchased from the Shanghai Baiye Biotechnology Center (China). HK-2 cells were cultured in DMEM medium (HyClone, USA) containing 10% fetal bovine serum (Zhejiang Tianhang Biotechnology Co. Ltd., China) at 37°C in a humidified 5% CO2 air atmosphere.
Lentivirus infectionsThe lentivirus encoding the human NRBP1 gene and green fluorescent proteins (GFP) were purchased from Shanghai GenePharma Co Ltd, China. The target cells were infected with the lentivirus according to the manufacturers’ instructions. The transfection effect 72h after infection was observed by fluorescence microscope camera, and the expression of NBRP1 was confirmed by western blot.
RNA interferenceOne short interfering RNA (siRNA) targeting the NRBP1 sequence (siNRBP1: GTCGAGAAGAGCAGAAGAA) was synthesized. siRNA targeting NRBP1 or scrambled siRNA (negative control, siCONT) were transfected using Lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA, USA) to knock down endogenous NRBP1. Total protein was collected from cells to analyze NRBP1 silencing efficiency.
Treatment of HK-2 cellsAfter the confirmation of NRBP1 overexpression and knockdown efficiency, HK-2 cells were assigned into 4 groups: control group (HK-2 cells cultured in normal DMEM medium), model group (HK-2 cells cultured in DMEM medium contain 400μmol/L uric acid), Lenti-NRBP1 group (HK-2 cells infected with the NRBP1 lentivirus and cultured in normal DMEM medium), and si-NRBP1 group (HK-2 cells were transfected by NRBP1 siRNA and cultured in DMEM medium contain 400μmol/L uric acid). After 48h of co-cultured, the further experiment was performed.
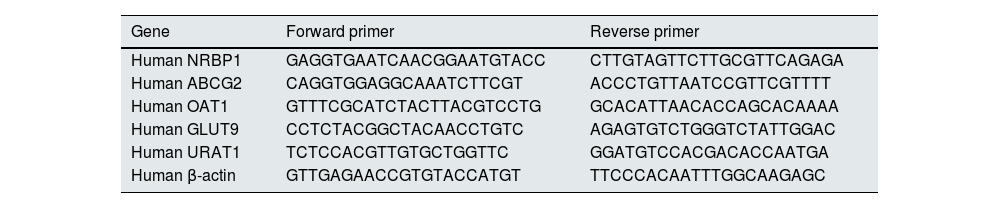
Quantitative real-time polymerase chain reaction (qRT-PCR)In order to determine the effect of NRBP1 overexpression and knockdown on the secretion and reabsorption of uric acid, the mRNAs expression of ABCG2, OAT1, GLUT9 and URAT1 were detected by qRT-PCR. Total RNA was extracted from each group using Trizol reagent (Bioengineering Company Limited, China) according to the manufacturer's instructions. Then it was reverse transcription to cDNA, amplified and analyzed using SYBR Green PCR Master Mix (Kangwei Century Biotechnology Co. Ltd, China) and a CFX96 real-time system (Bio-Rad, USA). Each group was analyzed in triplicate. The original threshold cycle (Ct) values were standardized with β-actin by the 2−ΔΔCt method. The primer sequences used are shown in Table 1.
Primer sequence of the genes for qRT-PCR analysis (5′-3′).
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Human NRBP1 | GAGGTGAATCAACGGAATGTACC | CTTGTAGTTCTTGCGTTCAGAGA |
| Human ABCG2 | CAGGTGGAGGCAAATCTTCGT | ACCCTGTTAATCCGTTCGTTTT |
| Human OAT1 | GTTTCGCATCTACTTACGTCCTG | GCACATTAACACCAGCACAAAA |
| Human GLUT9 | CCTCTACGGCTACAACCTGTC | AGAGTGTCTGGGTCTATTGGAC |
| Human URAT1 | TCTCCACGTTGTGCTGGTTC | GGATGTCCACGACACCAATGA |
| Human β-actin | GTTGAGAACCGTGTACCATGT | TTCCCACAATTTGGCAAGAGC |
To determine the cellular location of ABCG2 and its expression after NRBP1 overexpression and knockdoen, IF staining was performed. The treated HK-2 cells were fixed using 4% paraformaldehyde for 10min, washed with PBS three times, and permeabilized with 0.5% Triton X-100 (Sigma, St. Louis, MO, USA) for an additional 2min. After blocking with 3% bovine serum albumin (BSA) for 0.5h, ABCG2 was detected using mouse anti-human ABCG2 mAb (1:100, ab229193, abcam, USA) overnight. After washing, the cells were incubated with secondary antibody at room temperature. Finally, the cellular nuclei were stained by DAPI (40,6-diamidino-2-phenylindole) (Sigma, USA). The samples were observed under a fluorescence microscope (Nikon, Japan). The ImageJ software was used to quantify the fluorescence density.
Protein extraction and western blotThe efficiency of NRBP1 overexpression and knockdown, effects of NRBP1 overexpression and knockdown on the secretion and reabsorption of uric acid and Wnt/β-catenin signal pathway were all detected by western blot. Cellular protein was extracted from the cells transfected with or without siNRBP1 using RIPA lysis buffer (Beyotime Biotechnology, China). The protein concentration was detected using the bicinchoninic acid (BCA) protein quantification kit (Solarbio, Beijing, China). Equivalent amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred to polyvinylidene fluoride (PVDF) membranes. Then the protein blots were incubated with primary antibodies overnight at 4°C. After washing three times with TBST buffer and the protein blots were incubated with secondary antibody at room temperature for 1–2h. The reactive bands were visualized by use of the ECL-Plus reagent (Solarbio, Beijing, China). The density of band was quantified by the Quantity One analytic software. The primary antibodies including anti-beta Actin antibody (1:1000, ab8227, Abcam, USA), NRBP1 antibody (1:1000, DF10146, Affinity, USA), ABCG2 antibody (1:500, AF5177, Affinity, USA), Anti-SLC22A6 antibody (1:500, ab135924, Abcam, USA), Anti-GLUT9 antibody (1:500, ab223470, Abcam, USA), URAT1antibody (1:500, DF12340, Affinity, USA), Anti-beta Catenin antibody (0.25μg/mL, ab16051, Abcam, USA), GSK3 beta antibody (1:500, ab53050, Affinity, USA) and GSK3 beta antibody (1:500, ab53050, Affinity, USA).
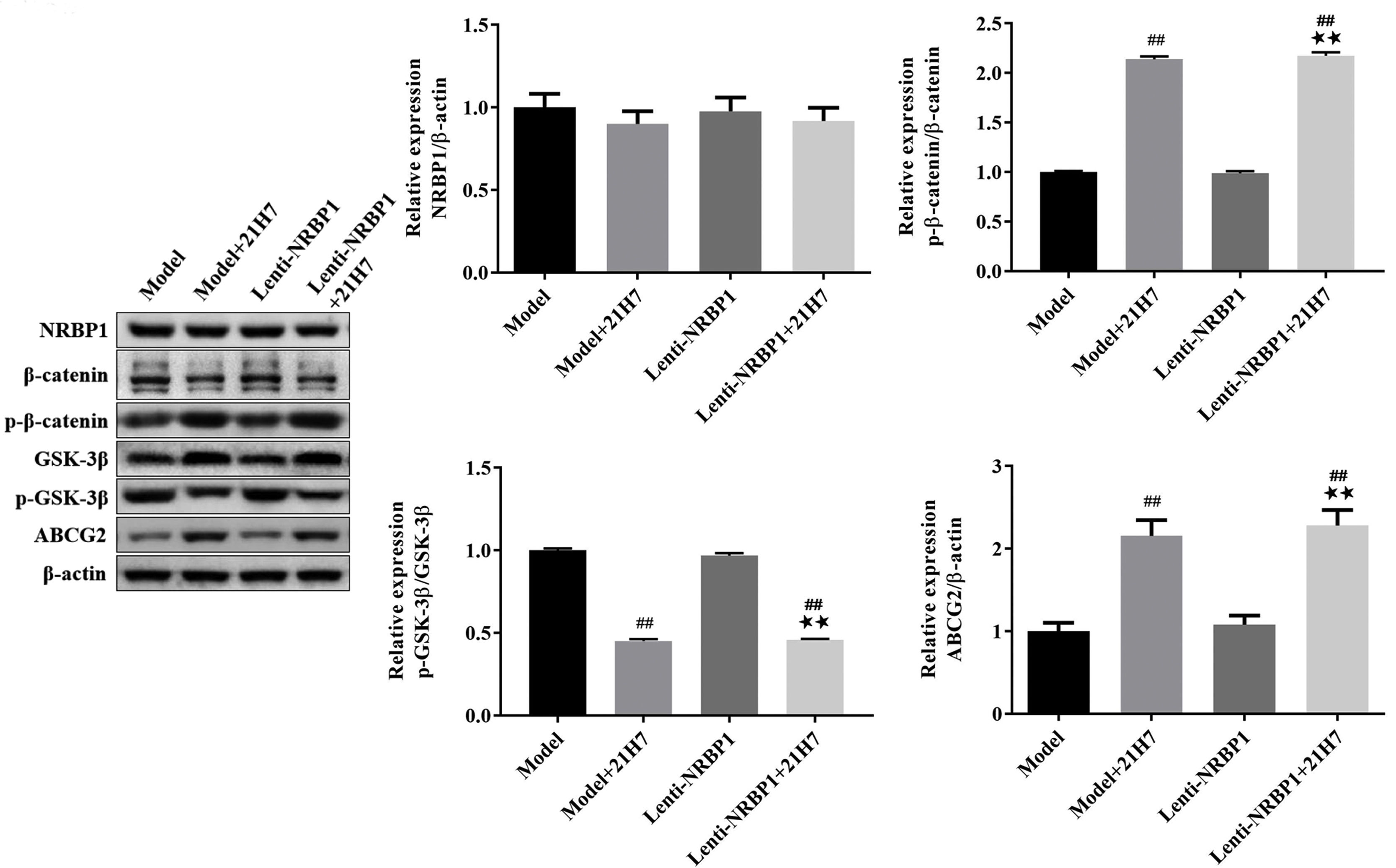
21H7 treatment21H7 is a selective inhibitor of the Wnt/β-catenin pathway that destabilizes β-catenin.23 The HK-2 cells were divided into four group: model group, model+21H7 group, Lenti-NRBP1 group and Lenti-NRBP1+21H7 group. After transfection with NRBP1 lentivirus, HK-2 cells were treated with or without 21H7 (4μM, Sigma-Aldrich, USA) for 48h.24 After that, the proteins expression of NRBP1, ABCG2, β-catenin, p-β-catenin, GSK-3β and p-GSK-3β were detected by western blot.
Statistical analysisThe statistical analyses were performed using SPSS 16.0 (IBM, Armonk, NY, USA). Values are expressed as χ¯±s. Comparisons of multiple independent groups were analyzed using One-way ANOVA followed SNK post hoc test. In all cases, P<0.05 was considered as statistical significance.
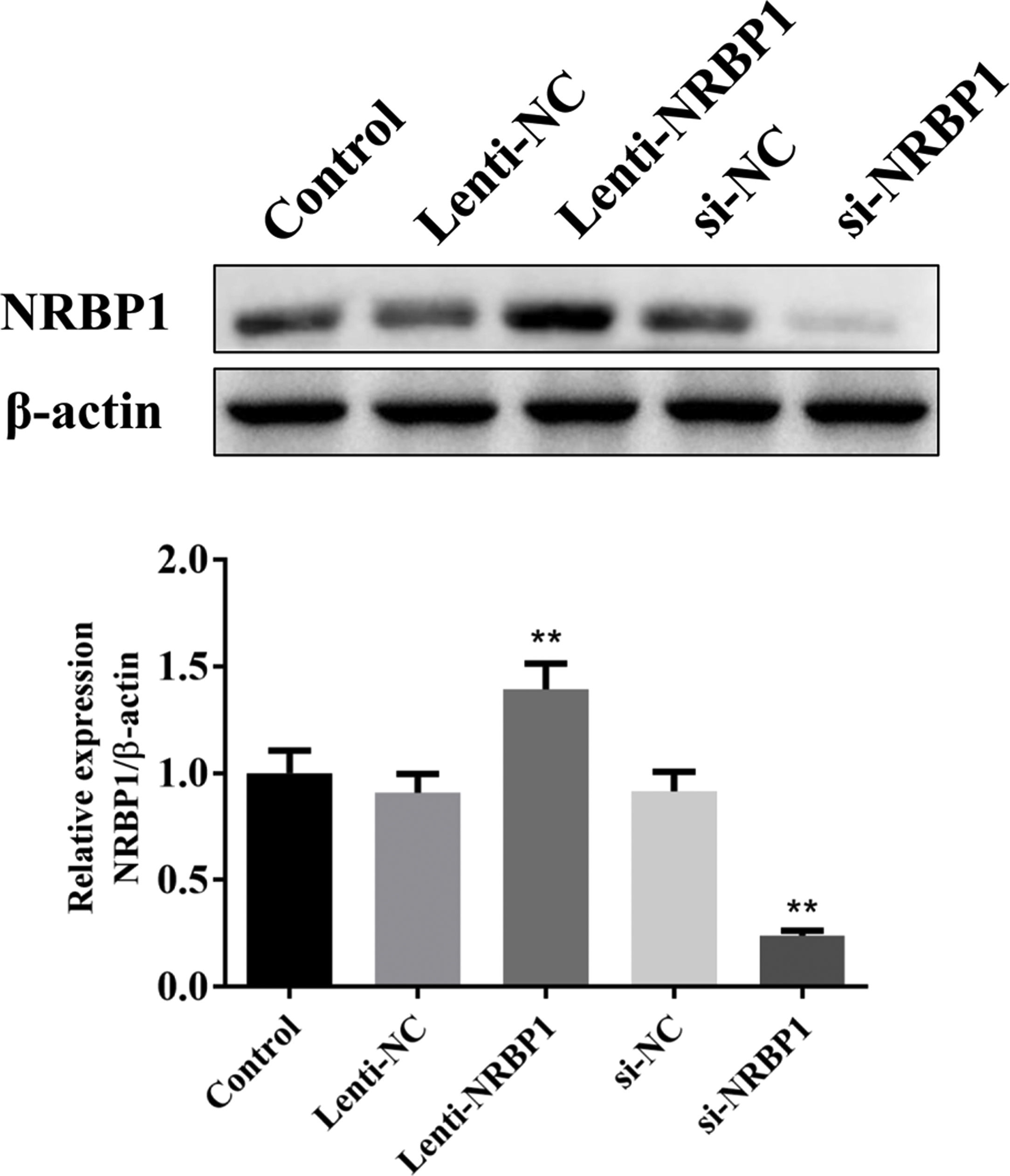
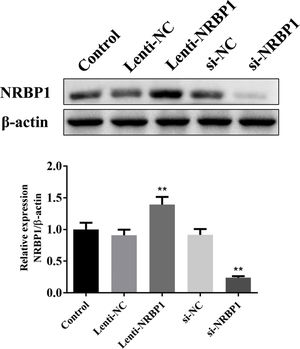
ResultsEfficiency of NRBP1 transfection and knockdownLentivirus system was used to generate stable NRBP1 overexpression in HK-2 cells. Western blot analysis showed that NRBP1 protein expression was significantly elevated in HK-2 cells transduced with Lenti-NRBP1 (P<0.01, Fig. 1). At the same time, plasmids carrying a NRBP1 siRNA was generated to knockdown NRBP1 expression in HK-2 cells. A reduced protein level of NRBP1 in HK-2 cells transduced with NRBP1 siRNA was verified by western blot (P<0.01). Taken together, the efficiency of NRBP1 transfection and silencing were significant.
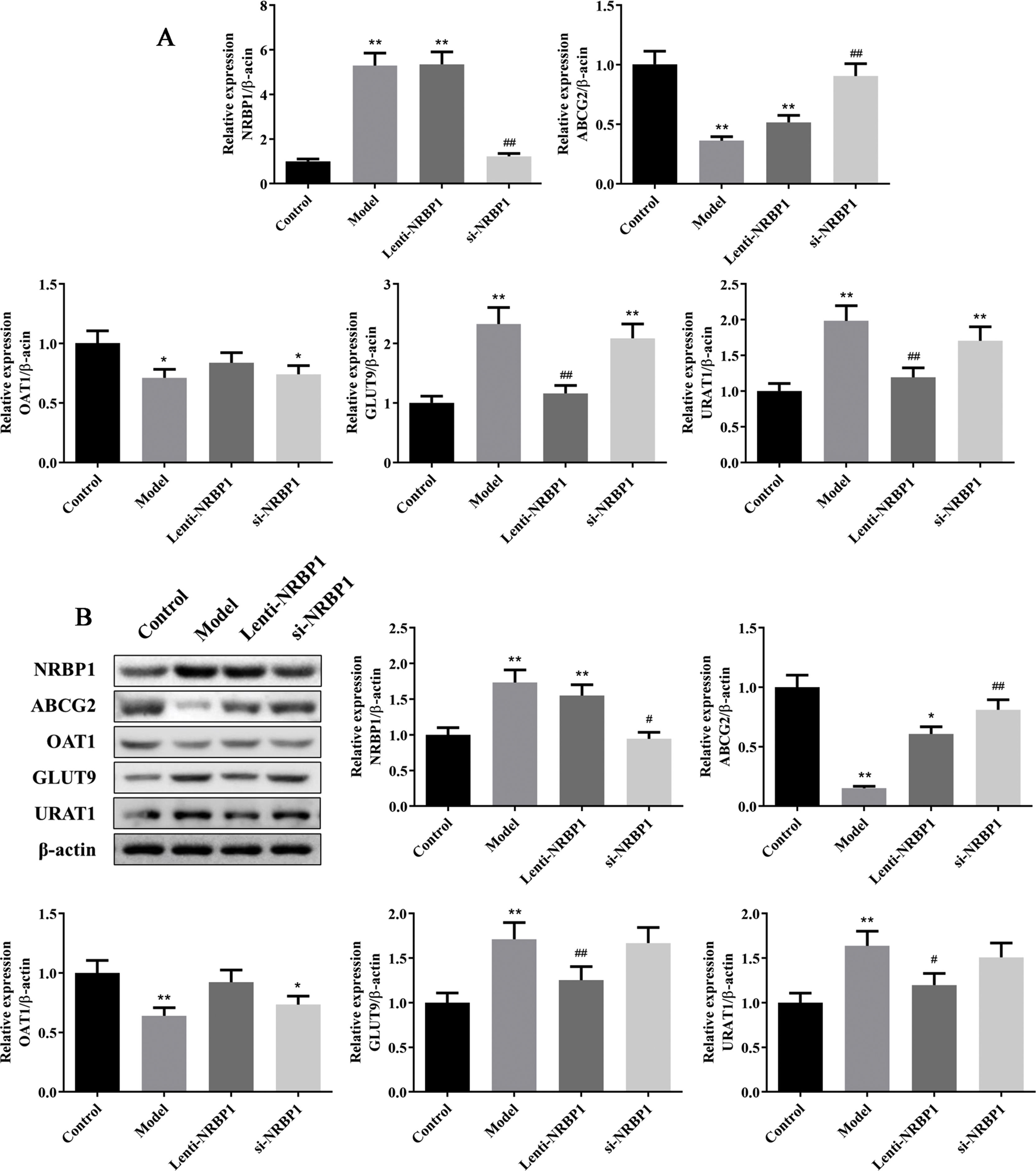
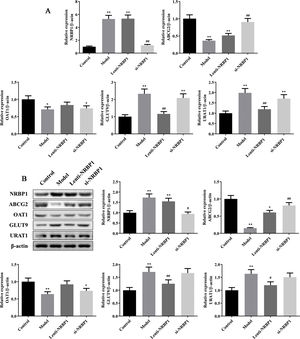
Effect of NRBP1 overexpression and knockdown on the secretion and reabsorption of uric acidThe mRNAs and proteins related to the secretion and reabsorption of uric acid such as ABCG2, OAT1, GLUT9 and URAT1 were detected by qRT-PCR and western blot. Compared with control group, the mRNAs and proteins expression of NRBP1, GLUT9 and URAT1 were significantly increased in model and si-NRBP1 group, while ABCG2 and OAT1 were decreased in model group (P<0.05, Fig. 2). Compared with model group, the mRNAs and proteins expression of GLUT9 and URAT1 was decreased significantly in Lenti-NRBP1 group (P<0.01). At the same time, the mRNAs and proteins expression of ABCG2 and NRBP1 in si-NRBP1 were significantly increased and decreased, respectively (P<0.01). Therefore, ABCG2 might be the target of NRBP1 for the treatment of hyperuricemia.
Effect of NRBP1 overexpression and knockdown on the secretion and reabsorption of uric acid. A, Effect of NRBP1 overexpression and knockdown on the mRNA expression of NRBP1, ABCG2, OAT1, GLUT9 and URAT1. B, Effect of NRBP1 overexpression and knockdown on the protein expression of NRBP1, ABCG2, OAT1, GLUT9 and URAT1. (χ¯±s,n=3), *P<0.05, **P<0.01 vs. Control group, #P<0.05, ##P<0.01 vs. Model group.
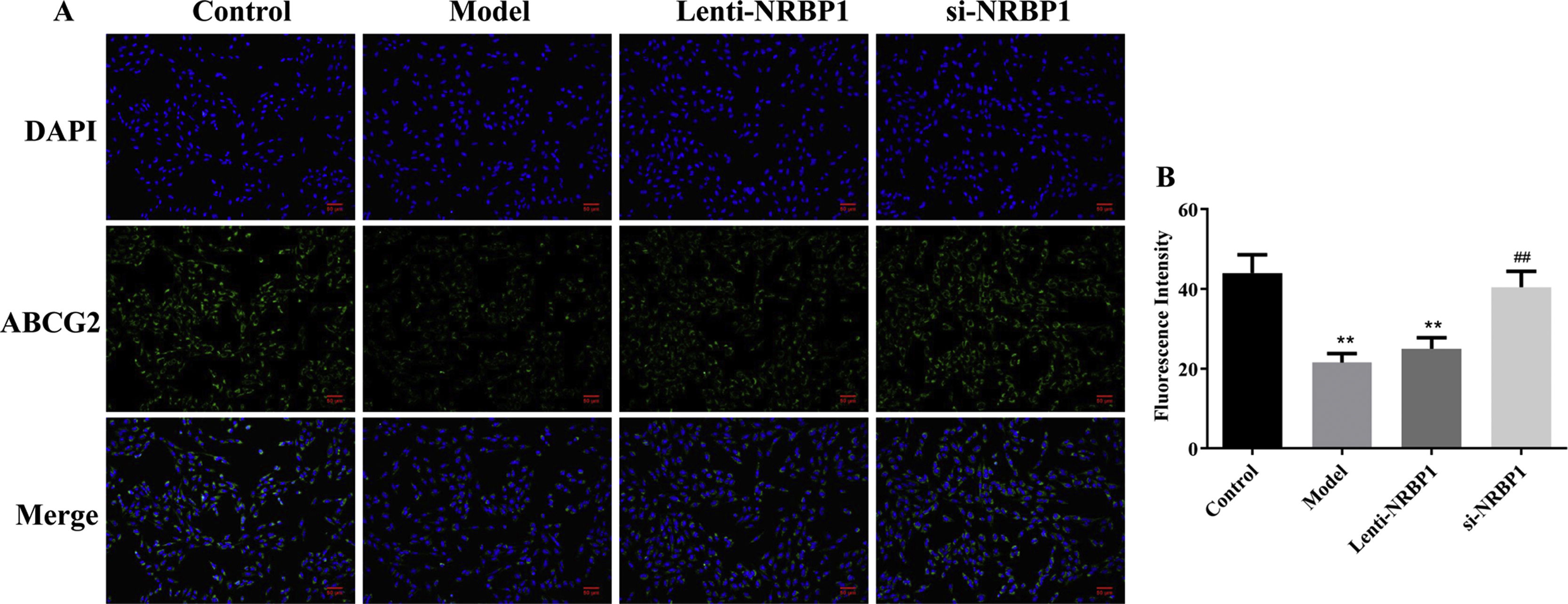
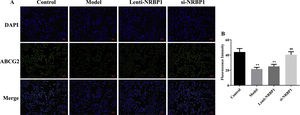
Because NRBP1 is a gout risk gene, we hypothesized that NRBP1 could regulate the ABCG2 mRNA and protein expression in proximal tubular epithelial cells, and then affect renal urate excretion. According to IF results (Fig. 3), NRBP1 overexpression inhibited the expression of ABCG2 in HK-2 cells (P<0.01). Compared with model group, the expression of ABCG2 was significantly increased in the si-NRBP1 group (P<0.01). The results provided compelling evidence regarding the inhibition effects of NRBP1 on the protein expression of ABCG2.
NRBP1 reduced the expression of ABCG2 protein level in HK-2 cells. A, Representative imaging from immunofluorescent staining (original magnification ×200). B, The relative fluorescent intensity for each group. (χ¯±s,n=3), *P<0.05, **P<0.01 vs. Control group, #P<0.05, ##P<0.01 vs. Model group.
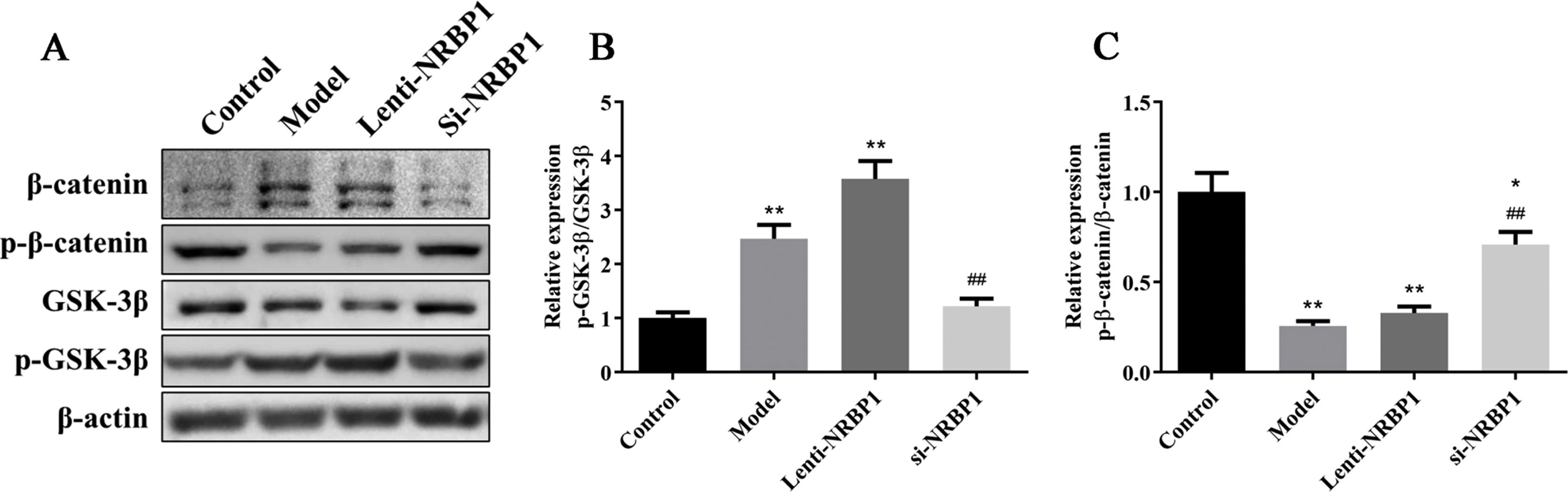
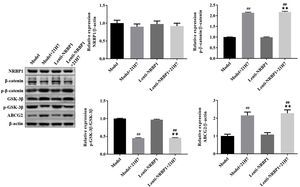
According to the western blot results (Fig. 4), the ratio of p-β-catenin/β-catenin was significantly upregulated in siNRBP1-transfected HK-2 cells compared with the model group (P<0.01). However, the ratio of p-GSK3β/GSK3β was significantly downregulated in siNRBP1-transfected HK-2 cells compared with the model group (P<0.01). In contrast, the ratio of p-GSK3β/GSK3β in stable NRBP1 overexpressing HK-2 cells was significantly upregulated (P<0.01), while the ratio of p-β-catenin/β-catenin was significantly downregulated (P<0.01), compared with the control group. Taken together, these findings suggested that NRBP1 may have a regulating role in the progression of renal urate excretion by activating the Wnt/β-catenin signaling pathway.
Effect of NRBP1 knockdown or overexpression on the Wnt/β-catenin signaling pathway in HK-2 cells. A, Western blot was used to analyze the expression levels of β-catenin, p-β-catenin, GSK3β and p-GSK3β in stably si-NRBP1-transfected or NRBP-1 overexpression HK-2 cells. B–C, Semi-quantification of the expression levels of proteins in parts A. (χ¯±s,n=3), *P<0.05, **P<0.01 vs. Control group, #P<0.05, ##P<0.01 vs. Model group.
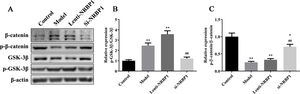
In order to further explored the effect of the Wnt/β-catenin pathway on NRBP1 mediated ABCG2 expression, the proteins expression of NRBP1, β-catenin, p-β-catenin, GSK-3β, p-GSK-3β and ABCG2 in HK-2 cells was performed by western blot after treated with the Wnt/β-catenin inhibitor 21H7. As shown in the Fig. 5, in the model+21H7 and Lenti-NRBP1+21H7 group HK-2 cells, the ratio of p-β-catenin/β-catenin and ABCG2 expression was markedly elevated (P<0.01), while the ratio of p-GSK-3β/GSK-3β was markedly decreased (P<0.01) compared with model and Lenti-NRBP1 group HK-2 cells. In addition, there was no influence on the protein expression of NRBP1 after blocked the Wnt/β-catenin pathway both in model and Lenti-NRBP1 group (P>0.05). These results suggested that NRBP1 modulates uric acid transporter ABCG2 expression by activating the Wnt/β-catenin pathway in HK-2 cells.
NRBP1 regulates uric acid transporter ABCG2 expression by activating the Wnt/β-catenin pathway in HK-2 cells. HK-2 cells were transfected with NRBP1 and cultured with 21H7 (Wnt/β-catenin-inhibitor), western blot was used to analyze the expression levels of NRBP1, β-catenin, p-β-catenin, GSK3β and p-GSK3β in HK-2 cells. (χ¯±s,n=3), #P<0.05, ##P<0.01 vs. Model group, ★P<0.05, ★★P<0.01 vs. Lenti-NRBP1 group.
At present, the classification of hyperuricemia is based on the understanding of its mechanism by which hyperuricemia results from either overproduction of urate due to a metabolism disorder, underexcretion by abnormal renal urate transport activity, or the combination of both.25,26 The main cause of hyperuricemia is ‘renal urate underexcretion’, and ‘urate overproduction’ is considered as another common cause.27,28 In the body, about 2/3 of urate is excreted by the kidney, while the other 1/3 of urate is excreted from the intestine into feces and broken down by colonic bacteria.29 Therefore, to improving uric acid excretion or inhibiting uric acid reabsorption via the kidney to reduce the level of uric acid might be a main effective approach to the prevention and treatment of gout and hyperuricemia.
A number of urate transport in the kidney regulate urate excretion and reabsorption to control uric acid balance in the blood.30 Among them, URAT1 and GLUT9 regulate uric acid reabsorption, while ABCG2 and OAT1 regulate urate excretion.31,32 The dysregulation of these urate transporters alters urate control in the body, increasing the risk for hyperuricemia.27,33 At present, the common dysfunctional variants of ABCG2 decrease extra-renal urate excretion including gut excretion and cause hyperuricemia.34,35
NRBP1 is a gout risk gene. In previous study, the hypomethylation at the promoter region of NRBP1 reduces the binding of TFAP2A and thus leads to elevated NRBP1 expression, which contribute to the development of gout.18 Before this, it has been showed that NRBP1 plays an important role in tumor suppression, cellular homoeostasis, and protein regulation by regulating the Wnt/β-catenin signaling pathway.36,37 Meanwhile, the urate transporters ABCG2 was regulated by Wnt/β-catenin signaling pathway.38 Interestingly, we found that HK-2 cells which overexpressed NRBP1 or NRBP1 knockdown can regulate the expression of ABCG2. However, the expression of NRBP1 had no significant effect on the expression of another urate transporters OAT1. In addition, compared with model group, the overexpression of NRBP1 also decreased the expression of GLUT9 and URAT1, but the NRBP1 knockdown had no significant effect on the expression of GLUT9 and URAT1. Therefore, we thought that NRBP1 overexpression decreased the expression of ABCG2 resulted in the accumulation of uric acid, further to induced gout or hyperuricemia, and illustrated that the uric acid transporter ABCG2 might be the target of NRBP1.
Therefore, we further explored whether the Wnt/β-catenin signaling pathway was involved in the regulation of NRBP1 on the expression ABCG2. In previous study has shown that NRBP1 was downregulated in breast cancer and its overexpression inhibits cancer cell proliferation through Wnt/β-catenin signaling pathway.19 Wnt/β-catenin signaling pathway also plays an important role in hyperuricemia nephropathy. The inactivation of the Wnt/β-catenin signaling pathway could induce the blockade of ERK1/2, and the inhibition of ERK1/2 could have therapeutic potential for treatment of hyperuricemic nephropathy.39 In this study, the ratio of proteins related to the Wnt/β-catenin signaling pathway, including p-β-catenin/β-catenin and p-GSK-3β/GSK-3β, were downregulated and upregulated respectively following NRBP1 overexpression, indicating that the Wnt/β-catenin signaling pathway may be activated. In contrast, following the knockdown of NRBP1, the ratio p-GSK3β/GSK3β was significantly downregulated in HK-2 cells, while the ratio p-β-catenin/β-catenin was upregulated, suggesting that the Wnt/β-catenin signaling pathway may be inhibited.
In addition, the inhibitor of β-catenin 21H7 was used to further explored the role of the Wnt/β-catenin signaling pathway in NRBP1 modulates uric acid transporter ABCG2 expression. In the model and Lenti-NRBP1 HK-2 cells, the addition of the β-catenin inhibitor 21H7 didn’t affect the expression of NRBP1, but increased the expression of ABCG2 and the ratio of p-β-catenin/β-catenin. Meanwhile, the ratio of p-GSK-3β/GSK-3β was significant. Thus, these results indicated that NRBP1 may serve a role in modulates uric acid transporter ABCG2 expression by activating the Wnt/β-catenin signaling pathway.
In conclusion, NRBP1 can inhibit the development of hyperuricemia by mediating the expression of ABCG2 to promote uric acid excretion via the kidney in HK-2 cells. Furthermore, NRBP1 was found to modulate uric acid transporter ABCG2 expression by activating the Wnt/β-catenin signaling pathway. The functional and mechanistic investigations suggested that the interactions between NRBP1 and GSK-3β, which may positively regulate β-catenin, may be the trigger for the activation of the Wnt/β-catenin signaling pathway in the modulates uric acid transporter ABCG2 expression. This study may provide pharmacological evidence to support the anti-hyperuricemic effect of knockdown NRBP1 in the treatment of hyperuricemia and gout.
Authors’ contributionsZaihua Zhu and Qiankun Zhang conceived and designed the study and performed the experiments and analyzed the data. Hang Fang contributed materials, data and assisted in data analysis; Qiankun Zhang and Hang Fang were the major contributor in writing the manuscript. All authors read and approved the final manuscript.
FundingThis study was supported by the funds from Project of the Natural Science Foundation of Zhejiang Province (Grant No.LY20H050009) to Qiankun Zhang and the National Natural ScienceFoundation of China (Grant No. 81701594) to Zaihua Zhu.
Conflict of interestAll authors declare that they have no conflict of interest.