En esta revisión nos gustaría realizar un resumen del conocimiento actual sobre la patogénesis y el tratamiento de la nefropatía isquémica. Los datos epidemiológicos sugieren que la prevalencia de la nefropatía isquémica aumenta, especialmente en los individuos de mayor edad. La patogénesis de esta enfermedad es más compleja que el mero estrechamiento de la arteria renal a causa de la ateroesclerosis. El sistema renina angiotensina, los factores de crecimiento, las diferentes citoquinas y las quimiocinas pueden estar implicados en la patogénesis de la nefropatía isquémica. Específicamente, los criterios diagnósticos de utilidad clínica de la nefropatía isquémica aún están por determinar. La gestión médica acordada de forma conjunta sigue siendo por el momento la principal opción terapéutica para la mayoría de los pacientes con esta patología y hoy en día, la revascularización está indicada únicamente en pacientes seleccionados.
In this review paper we would like to summarized the current knowledge concerning the pathogenesis and treatment of ischemic nephropathy. Epidemiological data suggest that the prevalence of ischemic nephropathy increases, especially among older individuals. The pathogenesis of this disease is more complex than just narrowing of the renal artery due to atherosclerosis. Renin-angiotension system, growth factors, different cytokines and chemokines may participate in the pathogenesis of ischemic nephropathy. Precise, clinically useful diagnostic criteria of the ischemic nephropathy have not been established, yet. Concerted medical management remains now the main therapeutic option for majority of patients with this disease and only in the selected patients revascularisation is nowadays indicated.
INTRODUCTION
Atherosclerotic renal artery stenosis (RAS) is often one of the sign of generalized atherosclerotic disease.1-3 Patients with atherosclerotic RAS are at a high risk of cardiovascular death.4 Conlon et al. found in patients who underwent cardiac catheterization, that 4 years survival was 86% in those subjects without RAS and only 65% in those with RAS.5 The degree of RAS does also significantly influence the survival. Four years survival in patients with RAS <75% of diameter was 89%, whereas in those with ≥75% narrowing survival was only 57%.6 Johansson et al. observed 164 patients with RAS >50% over 7 years.7 During this period 33 patients died and only in 2 patients the progression to stage 5 of chronic kidney disease (CKD) was observed. Risk of death in patients with RAS >50% was over 3 times higher than in general population.7 Therefore, it is important to stress that patients with ischemic nephropathy are more likely to die due to cardiovascular complications than to progress to CKD stage 5.
Atherosclerotic RAS can lead to the ischemic nephropathy (ischemic renal disease). Ischemic nephropathy is defined by the gradual reduction of the glomerular filtration rate (GFR) or a loss of renal parenchyma caused by vascular occlusion, not attributable to the other causes. Therefore, the ischemic nephropathy is characterized by a reduction of blood flow to the renal parenchyma beyond the level of renal autoregulation.
In patients with CKD stage 5 caused by ischemic nephropathy, survival during renal replacement therapy is also poor. Mailloux et al. observed a 25-months median survival and 18% 5 years survival rate in hemodialysis patients suffered from ischemic nephropathy which was significantly lower than 133 months median and 77% 5 years survival in hemodialysis patients with polycystic kidney disease.8
EPIDEMIOLOGY
The exact prevalence of ischemic nephropathy is difficult to estimate. The disease is often asymptomatic and few patients are screened unless they have clinical symptoms. Moreover, there are no precise, clinically useful and widely accepted diagnostic criteria of ischemic nephropathy.
Therefore, the percentage of patients who develop CKD stage 5 due to ischemic nephropathy is difficult to assess. Scoble et al. performed renal arteriography in all patients entering the hemodialysis programme.9 Ischemic nephropathy as a cause of terminal CKD was found in 6% of the entire group of patients.8 However, among patients older than 50 years ischemic nephropathy as a cause CKD stage 5 was observed in 14%.9 In the other studies ischemic nephropathy was diagnosed as a cause of CKD stage 5 in 11% and in 27% of patients respectively.10,11 Mailloux et al. in one centre study identified ischemic nephropathy as a cause of CKD stage 5 in 12.2% of patients entering the hemodialysis programme.12 In this study, ischemic nephropathy was the third greatest cause of CKD stage 5 after diabetes mellitus and chronic glomerulonephritis. Using the data from the United States Renal Data System, Fatica et al. reviewed the primary causes of CKD stage 5 and found that the incidence of ischemic nephropathy causing CKD stage 5 increased from 1.4% in 1991 to 2.1% per year in 1997.13 The risk of CKD stage 5 from ischemic nephropathy versus CKD stage 5 from other causes was positively correlated with the age of these patients and was higher in males. Result of this analysis may suggest that the prevalence of ischemic nephropathy is increasing during the recent years, especially among older, male individuals.
PATHOLOGY
The main macroscopic feature of ischemic nephropathy is the reduction of kidney size. In a study by Dean et al. a decreased kidney size over 10% was noted on serial intravenous pyelograms in 37% of RAS patients.14 Schreiber et al. examined in the retrospective study kidney length changes that were associated with arteriographic progression of RAS.15 Decrease in kidney size was defined as a >1.5cm difference in pole-to-pole measurements on serial x-ray pictures. Forty four percent of patients with atherosclerotic RAS have demonstrated the disease progression and 70% of these patients showed significant decrease of kidney size.15 Caps et al. showed the reduction of kidney size during two years observation period in 20.8% kidneys with ≥60% RAS.16
It is not completely clear whether reduced kidney size always indicates irreversible parenchymal changes. In the histology of ischemic nephropathy both reversible tubular atrophy and glomerular collapse and irreversible glomerulosclerosis and interstitial fibrosis are present. Therefore, only result of renal biopsy is able to distinguish between the above mentioned causes of kidney atrophy, but it is rarely done in these settings because of the procedural risk of complications and no clear therapeutic implications.
The earliest and the most pronounced histopathologic feature of the ischemic nephropathy are tubulointerstitial lesions.17,18 In 62 patients with RAS who underwent nephrectomy of a small kidney for uncontrolled hypertension, the predominant pattern of injury was significant tubulointerstitial atrophy with relative glomerular sparing.19 Tubuloinsterstitial lesions may mediate the CKD progression towards irreversible damage. At the early phase of this process, cellular activation takes place, as mononuclear cells migrate into the interstitium and myofibroblasts release inflammatory mediators that lead to inflammation.20 Apoptosis induces tubular atrophy which also occurred in patients with ischemic nephropathy.21 This phase of disease may still be partly reversible until fibrosis begins.22 Subsequent fibrosis leads to the kidney permanent damage.
Glomerulosclerosis is a late event in patients with ischemic nephropathy. Glomerular lesions are initially minimal in experimental animals with chronic ischemic nephropathy and observed only when severe stenosis is present.17,19,23,24 In some patients glomerular collapse with loss of tuft volume due to hypoperfusion is observed. Moreover fibrosis in the interstitial tissue in the vicinity of Bowman capsule of glomerulus can obstruct the origin of proximal tubuli and may lead to development of atubular, nonfunctioning glomeruli.
In addition to tubulointerstitial injury, in patients with ischemic nephropathy atheroembolic intrarenal disease or severe small vessel disease are frequently observed. Keddis et al. in histopathological examination found that intrarenal atheroembolism were present in 39% of small kidneys nephrectomized due to uncontrolled hypertension.19 Renal microvascular disease in patients with ischemic nephropathy may lead to vascular rarefaction within interstitium (reduced number or length of peritubular capillaries) and to increase in vascular wall/lumen ratio.25,26 The cause of these lesions is prolonged periods of vasoconstriction which leads to permanent structural changes in the microcirculation.27 Since microvascular remodeling correlates with the renal scarring, it may have an important implication on CKD progression in atherosclerotic renovascular disease.28
The severity of histopathological damage in renal parenchyma is an important determinant of renal outcome in patients with ischemic nephropathy. Wright et al. investigated the impact of histological lesions on renal functional outcome in a small group of patients whose renal biopsies suggested ischemic nephropathy, irrespective of whether significant RAS has been demonstrated in angiography.29 The authors found a tight relationship between decreases in estimated GFR (eGFR) over time and renal damage score.29
PATHOGENESIS
The pathogenesis of ischemic nephropathy is more complex than just narrowing of the renal artery from atherosclerosis. In contrast to the results from the animal experiments (for example Goldblatt hypertension in dogs induced by clamp to the renal artery) in which RAS is accompanied by the intact renal vascular tree, in humans with atherosclerotic RAS the renal vasculature is often damaged. Therefore in animal model GFR decrease due to RAS is the reversible phenomenon. In contrast, in the majority of patients with atherosclerotic RAS, due to irreversibility of vascular and interstitial lesions, GFR decrease is irreversible.
Although ischemic nephropathy is caused by RAS, the relationship between RAS intensity and presence of ischemic nephropathy or the severity of renal dysfunction is weak. In a study of 71 patients with atherosclerotic RAS, Suresh et al. found that renal function was equally decreased in patients with mild proximal renovascular disease as in those with severe RAS.30 Similarly, Cheung et al. assessed time of progression to CKD stage 5 in patients with unilateral atherosclerotic renal artery occlusion and contralateral <50% or >50% RAS.31 Irrespective of whether the nonocluded artery was normal or had stenosis of varying significance, time to starting renal replacement therapy or death did not correlate with renovascular anatomy.31 The most likely explanation of these observations is that decrease of GFR in patients with RAS is mainly not determined by severity of RAS, but to the extend kidney lesions downstream of the RAS. It seems that reduction of the kidney length may be a better indicator of progression of ischemic nephropathy than the degree of RAS. As much as renal atrophy can be attributed to lack of blood flow, changes in kidney size seems to be more reasonable outcome measure of the effect of reduction of blood flow.
The kidneys physiologically receive relative blood supply three to five fold higher than heart or liver. It seems that in kidneys perfusion pressure below 70-80mmHg (correlates with >70% of RAS) overcome adaptive mechanisms and lead to hypoxia. However pathological changes do not appear to be directly related to the parenchymal tissue hypoxia. Patients with ischemic nephropathy are heterogeneous with respect to the degree of hypoxia in kidney tissue. In the early stages of ischemic nephropathy renal hypoxia in viable tissues seems to be present. Experimental study in pigs, showed during acute RAS, a decrease of regional intrarenal tissue oxygenation (measured directly with oxygen electrodes). Result of this experimental study confirmed the existence of hypoxia in kidney tissues.32
In contrast to this experimental observations, some recently performed clinical studies in the later stages of the disease, did not confirm the presence of hypoxia in the kidney tissue of patients with ischemic nephropathy. Renal tissue oxygenation was analyzed in patients with RAS by blood oxygen level-dependent magnetic resonance imaging (BOLD-MRI) technique. This technique uses the paramagnetic properties of desoxygenated hemoglobin. During oxygen extraction from the blood, increasing tissue concentrations of desoxygenated hemoglobin led to a decrease of transverse relaxation time (T2*) and an increase in the rate of spin dephasing (R2*).33,34 Based on the estimation of these parameters, tissue oxygenation can be visualized. With this technique Gloviczki et al. studied patients with RAS accompanied by reduction volume of kidney downstream to RAS.35 In this study, they demonstrated preserved medullary and cortical tissue oxygenation. It may be caused by the decrease of oxygen consumption by failed kidney. In the other study, measurement of oxygen tension in the renal veins of patients with RAS, also did not demonstrate desaturation.36
The preserved tissue oxygenation in kidney with RAS is manifested by normal plasma erythropoietin concentration in its renal vein.37 Based on the above mentioned data, it may be proposed that in the early stages, renal hypoxia plays a crucial role in the pathogenesis of ischemic nephropathy. However, in the later stages, tubulointerstitial fibrosis dominates.38
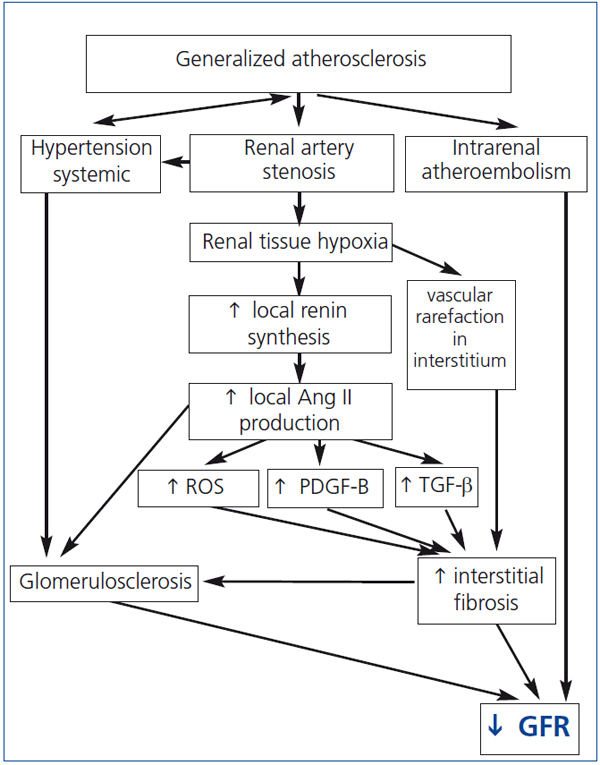
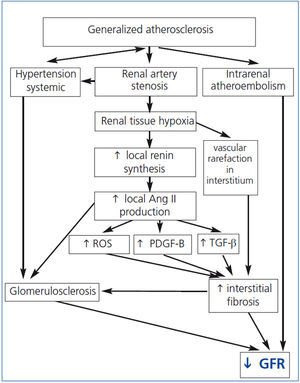
Renal hypoperfusion, leads to increased secretion of renin and generation of angiotensin II (Ang II).39 Recent studies confirmed that stenosis must be advanced in order to determine the hemodynamic effect. Careful studies using expanded balloons in humans indicate that an aortic–renal gradient exceeding 25 mmHg is necessary to increase renin secretion.39 This coincides with cross-sectional vascular occlusion approaching 70–80%. Ang II increases the expression of the transforming growth factor β (TGF-β) and platelet-derived growth factor-B (PDGF-B), resulting in the accumulation of extracellular matrix and collagen type IV in the perivascular tissue, interstitium and finally in the glomeruli (Figure 1).22,40
The progressive kidney fibrosis may occur also through the other mechanisms of tissue and vascular injury - aldosterone–mediated damage, sympathetic overactivity and increased release of reactive oxygen species (ROS).
Ang II stimulates the production of ROS. Lerman et al. demonstrated increased oxidative stress in pig model of RAS and Higashi et al. and Minuz et al. in patients with RAS.41-43 ROS increases renal vascular tone, sensitivity to vasoconstrictors and leads to endothelial dysfunction.44 The interaction of ROS with nitric oxide (NO) decreases bioavailability of the latter and results in formation of the prooxidant peroxynitrite. Reduction of NO activity allows vasopressors like angiotensin II and endothelin-1 (ET-1) to dominate and to lead to vasoconstriction and as a consequence to GFR decrease.
Ischemic nephropathy is characterized by the endothelial disfunction. The importance of the endothelial damage in ischemic nephropathy was demonstrated in an experimental porcine model. A single intrarenal infusion of autologous endothelial progenitor cells (EPC) preserves microvascular architecture and function and decreases microvascular remodeling.45
CLINICAL PICTURE AND DIAGNOSIS
The clinical presentation of patients likely to develop ischemic nephropathy consist of: older age (over 50 years), hypertension (usually severe or difficult to treat), history of smoking, hypercholesterolemia, coronary heart disease or the other clinical manifestation of atherosclerosis. Such a patient has gradually decreasing GFR, mild to moderate proteinuria and bland urinary sediment. Loss of GFR, as in the other nephropathies, may be an useful parameter for progression of ischemic nephropathy.
Proteinuria may also be recognized as a marker of severity of ischemic nephropathy. Makanjuola et al. surveyed 94 patients with atherosclerotic RAS. Fifty two percent of the patients had proteinuria. In patients with GFR ≥50ml/min mean urinary protein excretion was 400mg/24h, and in patients with GFR <50ml/min proteinuria ranged from 500mg/24h to 2.4g/24h.46
It is important to stress that precise, clinically useful diagnostic criteria of ischemic nephropathy have not been established, yet. The diagnostic evaluation of patients with suspected ischemic nephropathy is similar to the evaluation of patients with presumed RAS.
TREATMENT
At the present time concerted medical management (includes lifestyle modifications – among others smoking cessation, antihypertensive drugs, statins and antiplatelet treatment) remains the main treatment option for all patients with ischemic nephropathy. The optimal blood pressure in patients with ischemic nephropathy is unknown. Recent experimental studies suggested that novel approaches to treat the renal parenchyma directly, such as antioxidants, statins, VEGF and EPC may improve renal injury even without correcting the RAS.45,47-50
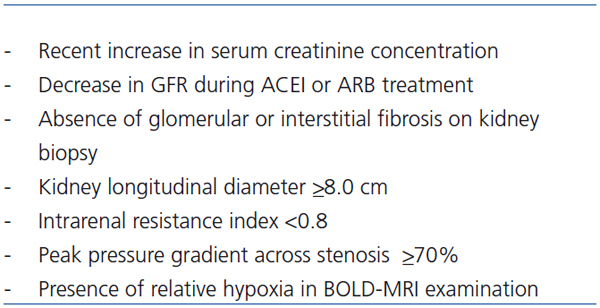
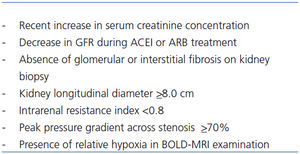
There is also a great need to identify accurately those individuals with ischemic nephropathy who will derive clinical benefit from renal revascularization. It is thought that only in the selected patients revascularisation is indicated.51 Exclusively patients with ischemic nephropathy with potentially reversible both interstitial and glomerular lesion may benefit from revascularisation. These patients should be characterized by only limited interstitial fibrosis and mainly no sclerotic glomeruli. Currently there are no good and clinically useful markers which allow to identify such patients i.e. there are no reliable tools to predict the reversibility of ischemic lesions in the kidney. In order to do it with contemprorary available methods the following strategies were proposed (Table 1).51
1/ Measurement of the kidney length by the ultrasound examination. Small cirrhotic kidney (longitudinal diameter less than 8.0cm) suggests advanced kidney fibrosis. However patients with concomitant kidney disease as diabetic kidney disease or amyloidosis may present normal kidney size despite advanced fibrosis. Moreover it is not completely clear whether reduced renal size always indicates irreversible parenchymal changes. There are series of patients with good renal functional outcome after revascularisation besides found before this procedure renal atrophy.52
2/ Assessment of the intrarenal resistance by Doppler sonography. Resistance index (RI) higher than 0.8 suggests advanced kidney fibrosis.53 However, the predictive value of increased RI is not unanimously accepted.54
3/ Measurement of the pressure gradient across a stenosis of the main artery invasively during arteriography. The gradient is directly proportional to the resistance in the renal vasculature downstream. The peak pressure gradient ≥70%, may predict that stenosis itself is hemodynamically important and influences the blood kidney supply and give a hope for reversibility of the interstitial and glomerular lesions.
It should be stressed that none of the above mentioned methods was validated in the properly controlled clinical studies. All of this tests are to some extend inadequate in predicting which patient with ischemic nephropathy will benefit from revascularization. Because of clinical applicability and non invasive character both length kidney measurement, by the ultrasound examination and assessment of the intrarenal resistance, by Doppler sonography were currently broadly used.
In future BOLD-MRI may also be useful for prediction the reversibility of ischemic lesions in the kidney. Presence of local ischemia hypothetically may predict reversibility of the changes. BOLD-MRI allows to analyze the patterns of regional tissue oxygenation in ischemic kidneys. This technique uses the paramagnetic properties of desoxygenated hemoglobin. During oxygen extraction from the blood, increasing tissue concentrations of desoxygenated hemoglobin led to a decrease of transverse relaxation time (T2*) and an increase in the rate of spin dephasing (R2*).33,34 Two diagnostic approach with the use of BOLD-MRI techniques were studied in patients with RAS: BOLD-MRI measurements combined with isotopic single kidney glomerular filtration rate (isoSK-GFR) and BOLD-MRI before and after furosemide treatment.55,56
Chrysochou et al. in preliminary, prospective pilot study showed that BOLD-MRI measurements combined with isoSK-GFR may be prognostic markers of renal functional recovery after revascularization. They showed that high R2*:isoSK-GFR ratio predicts a renal recovery after revascularization.55 The ratio of R2*:isoSK-GFR reflects metabolically active, hypoxic renal tissue, that is, the presence of potentially salvageable renal tissue. Such kidney parenchyma has not yet been subject to the deleterious cascade of ischemic events that results in irreversible structural changes.
Textor et al. have shown that furosemide (which inhibits medullary tubular sodium transport and oxygen consumption) decreases medullary deoxyhaemoglobin concentration (measured by R2*) in patients with normal-sized kidneys downstream to a high-grade RAS.56 In the atrophic kidneys distal to total occlusion, furosemide did not decrease deoxyhaemoglobin concentration. It suggest low kidney tissues viability. Therefore R2* mapping may distinguish between severely compromised but viable parenchyma (high basal values of R2* which would fall after the administration of furosemide) present in those kidneys likely to improve after revascularization and non-functional scarred tissue (low basal values of R2*, unaffected by furosemide).56
Currently, in daily clinical practice it is thought that revascularisation in patients with ischemic nephropathy is not indicated in several clinical situation like: a) in patients with normotension or when normal blood pressure is obtained with antihypertensive therapy, b) when stenosed renal artery is supplying small cirrhotic kidney (longitudinal diameter less than 8.0cm in adult patient) c) in patients with high intrarenal resistance assessed by Doppler sonography (RI higher than 0.8) corresponding to the advanced kidney fibrosis due to chronic ischemic nephropathy.
Key concept: The prevalence of ischemic nephropathy seems to increase, especially among older individuals. It’s pathogenesis is more complex than just narrowing of the renal artery from atherosclerosis. Precise, clinically useful diagnostic criteria of ischemic nephropathy have not been established, yet. Concerted medical management remains now the main therapeutic option for majority of patients with this disease and only in the selected patients revascularisation is nowadays indicated.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Table 1. Predictors of salvageability of ischemic nephropathy after revascularisation
Figure 1. Pathogenesis of ischemic nephropathy