El mecanismo de regulación de los niveles de Parathormona (PTH) es complejo, y en él intervienen diversos factores: los fundamentales son el calcio, el calcitriol y el fósforo. El mecanismo de acción de calcio y calcitriol tiene lugar a través de sus receptores específicos, el Receptor-sensor de Calcio (CaR) y el Receptor de Vitamina D (VDR). Estos dos factores tienen efecto no sólo sobre sus receptores específicos sino que pueden modificar en sentido positivo al otro receptor, potenciando sus acciones y demostrando un efecto cooperativo entre ambos. Además de calcio y calcitriol, los fármacos que se utilizan en el tratamiento de las alteraciones del metabolismo óseo y mineral de la Enfermedad Renal Crónica (ERC) también actúan directa o indirectamente sobre CaR y VDR y, por tanto, también son responsables de la regulación de la paratiroides.
The mechanism of regulation of Parathyroidhormone (PTH) is complex, and diverse factors are involved: the fundamental ones are calcium, calcitriol and phosphorus. Calcium and calcitriol's mechanism of action takes place through its specific receptors, the calcium-sensing receptor (CaR) and the Vitamin D Receptor (VDR). These two factors have an effect not only on its specific receptors, but also they can modify the other receptor in a positive manner, promoting its actions and demonstrating a cooperative effect between the two. Along with calcium and calcitriol, drugs used in the treatment of Chronic Kidney Disease Mineral Bone Disorders (CKD-MBD) also act directly or indirectly on CaR and VDR and therefore are also responsible for the regulation of the parathyroidgland.
INTRODUCTION
CKD progression implies the initiation of multiple compensating regulatory mechanisms, such as stimulation of the parathyroid gland with consequent increase of circulating levels of PTH. For years, a series of factors have been identified in the development of secondary hyperparathyroidism, which could also be responsible for the increase of morbidity and mortality observed in dialysis patients.1-3
Regulation of PTH levels is controlled through a complex feedback mechanism, in which levels of ionic calcium,4 calcitriol5 or its derivatives6,7 and low levels of phosphorus8 inhibit PTH secretion. Other factors have also been described, which can act on the parathyroid gland modifying PTH secretion and/or synthesis, such as aluminium,9 oestrogens,10 magnesium, corticosteroids and certain cytokines.11 More recently, Fibroblast Growth Factor 23 (FGF-23) has been added to this list, which is capable of directly inhibiting PTH synthesis and secretion.12
The parathyroid gland is regulated via its receptors, among which, CaR, VDR, and fibroblast growth factor receptor (FGFR) stand out. Although many regulatory processes are found to be specifically mediated by these receptors, their role and signalling mechanisms are controversial. The effects of calcium, calcitriol and even phosphorus on parathyroid function take place through specific mechanisms; however, there are also indirect actions which depend on the close connection and interrelation among calcium, calcitriol and phosphorus on the calcium and vitamin D receptors involved in the parathyroid regulation. In this article, we intend to critically revise and update the role of CaR and VDR receptors in PTH production.
CALCIUM AND CALCIUM RECEPTOR
Extracellular calcium ion is the main parathyroid regulator.13 Low levels of calcium stimulate PTH secretion in a few minutes, while elevated levels inhibit hormone release, and favour degradation within parathyroid cells themselves.14 This results in a sigmoidal parathyroid gland response, in which small changes of extracellular calcium ion cause large PTH variations, acquiring its greatest inhibition in hypercalcaemia.
The effects of calcium on PTH are mediated by its specific receptor, CaR,15 which belongs to the family G-protein-coupled receptors, it is presents on the membrane of the parathyroid cells. An increase in extracellular calcium is sensed by CaR, which triggers a cascade of intracellular signalling that results in the inhibition of PTH secretion and synthesis.
Although the CaR expression, just as its RNA messenger (MRNA) and its protein product, can be altered in many circumstances. In the majority of cases the reasons by which this occurs have not been clarified. Various studies have observed a dramatic decrease in CaR expression in monolayer or dispersed parathyroid cell cultures,16,17 but not when glands are cultured in whole, fragmented or laminated fashion.18 In view of these results, and although the underlying mechanism is unknown, it seems that CaR expression depends on the tissue’s three-dimensional structure. Indeed, when disperse parathyroid cells are cultured in a collagen matrix that allows their regrouping in a form resembling parathyroid tissue (pseudogland), the receptor’s expression recovers.19
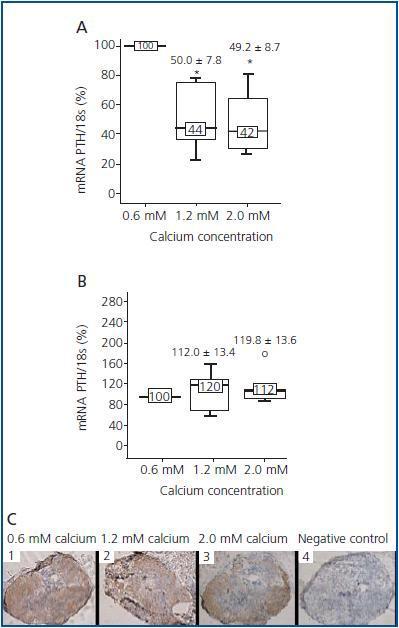
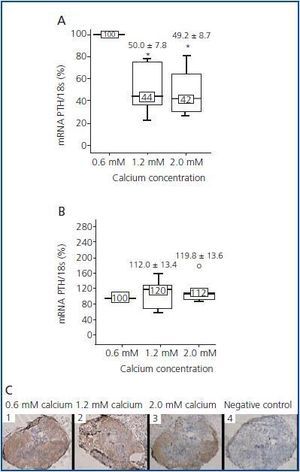
In addition, although the principal action of the CaR is to sense calcium, CaR expression and concentration in parathyroid glands do not seem to depend on extracellular levels of calcium. In vivo studies have shown that animals fed up with a diet high or low in calcium do not show differences in CaR levels in parathyroid glands, suggesting that calcium does not have a regulating effect on its receptor.20,21 Nevertheless, when interpreting and analyzing the results, it is fundamental to take into account that in in vivo studies, variations in one of the factors regulating PTH can also induce changes in other factors that are also capable of regulating PTH, therefore concealing or disturbing the true effect of the factor under investigation. In a recent study by our group,22 we tried to tackle these questions by modifying only one of the regulators and maintaining the rest constant. Thus, we demonstrated in parathyroid glands of normal rats that increasing calcium concentrations lowered PTH mRNAlevels, but not CaR expression, indicating that this effect was due to the activation of the receptor but not to increase in its levels. During the 24 hours of culture with the different concentrations of calcium, CaR mRNA and protein levels did not change even when doubling the concentration of calcium present in the culture medium (figure 1).
CALCITRIOL AND VITAMIN D RECEPTOR
Calcitriol is also an important parathyroid gland regulator and exerts a direct effect on PTH secretion by inhibiting of its mRNA synthesis.14
Calcitriol acts on the parathyroid gland through its specific receptor, VDR, a high affinity and specificity receptor, which belongs to the steroid/thyroid receptor group. When calcitriol binds its receptor, it produces the translocation of the calcitriol- VDR complex to the cell nucleus, forming a heterodimer with the Retinoid X Receptor (RXR). Calcitriol-VDR-RXR complex binds Vitamin D Responsive Elements (VDRE) present in the PTH gene promoter region, blocking its transcription. In addition to this, calcitriol is capable of indirectly inhibiting PTH secretion by augmenting calcium absorption in the intestine, and at the same time stimulating bone resorption and calcium removal.23
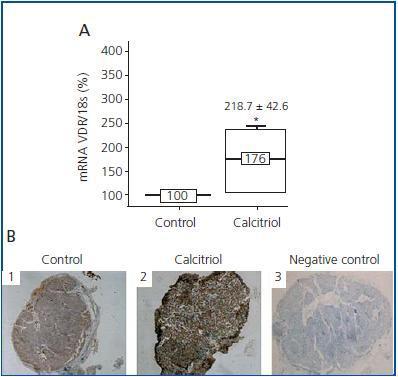
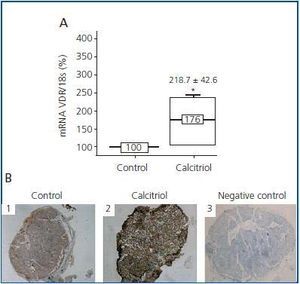
Contrary to what occurs with calcium and CaR, calcitriol regulates the expression of its own receptor (VDR), stimulating its synthesis24 and increasing its average life.25 Therefore, the calcitriol deficit observed in patients with CKD is associated with a decrease in VDR levels in the parathyroid gland.26 Until now, all studies on calcitriol’s effect on VDR levels have always been carried out in vivo,27-29 and it has been observed that calcitriol regulation of VDR had only been effective in hypercalcaemic and normocalcaemic conditions, not in hypocalcaemia suggesting that calcium levels may be critical for controlling VDR levels. However, in a recent study22 in which parathyroid glands were cultured in low levels of calcium, we observed that calcitriol was capable of increasing levels of both VDR mRNA and protein levels (figure 2), suggesting less VDR dependence in relationship with calcium concentration.
COOPERATION AMONG CALCIUM, CALCITRIOL, CaR AND VDR
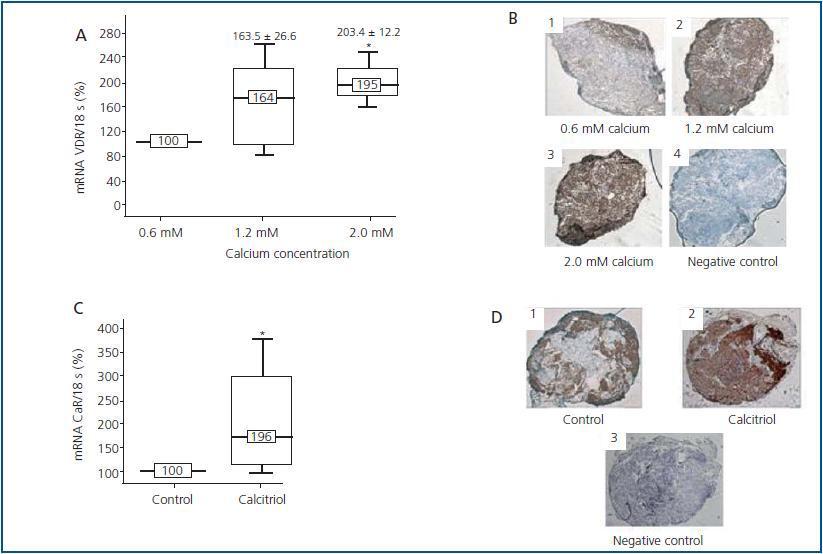
Besides the described effect of calcium and calcitriol on its own receptors, both can cooperate by modifying each other’s response in a positive manner. Even though VDR is the specific receptor for calcitriol, and other metabolites analogues of vitamin D, calcium is also capable of regulating VDR levels. Diverse in vivo28,29 and in vitro22,29 studies have described this effect, such as the recently mentioned study by our group22 in which it was observed that after 24 hours culture of parathyroid glands with calcium, levels of VDR mRNA and protein (figure 3A y 3B) increased.
It has also been described that calcitriol can also regulate CaR, although in this case results are still contradictory. Some studies did not detect variations in the levels of CaR with calcitriol.21 Others only find a CaR increase in normal and hypercalcaemic conditions,20,29 whilst other investigators have observed this effect even when the parathyroid glands are cultured with a low concentration of calcium.22 In these conditions, it has been shown that calcitriol, after 48 hours culture, increases not only the CaR mRNA but also its protein (figure 3C y 3D). It has been speculated that this effect of calcitriol on CaR could be mediated through VDRE, also present in the CaR gene promoter.30
OTHER MODULAR FACTORS OF CaR AND VDR
In addition to the regulation that calcium and calcitriol exert over their receptors, there are other factors and drugs which can regulate CaR and VDR levels. One of these factors is phosphorus, that apart from exerting direct regulation of PTH by increasing its secretion,31 it can exert an indirect regulation, modifying the CaR and VDR expression, although this topic is debated. In the case of CaR, some studies have shown that a high phosphorus diet is capable of reducing CaR’s expression,32-34 although previous in vivo work did not detect changes in CaR expression in parathyroid glands with diets high or low in phosphorus.35,36
Furthermore some investigators have demonstrated that phosphorus could modify VDR expression. This effect appears to be tissue-specific, given that in the intestine phosphorus could increase VDR expression while it could lower it in the kidney.37 It is important to emphasize the role of the phosphate binders here. The hyperphosphataemia characteristic of the advanced stages of CKD, could be responsible for the variations in CaR and VDR levels in parathyroid glands. The use of phosphate binders to reduce hyperphosphataemia could help normalizing CaR and VDR expression levels.
Aluminium is another factor capable of regulating the parathyroid function by inhibiting PTH secretion that could intervene in CaR and VDR regulation.38 Previous studies by our group have demonstrated the existence of an aluminium dose-dependent inhibitory effect on PTH mRNA levels in rats with chronic renal failure,39 by reducing CaR gene expression through a posttranscriptional mechanism.9 However, it is unknown whether aluminium has any effect on VDR.
Finally, calcimimetics, which modulate CaR allosterically, increase the receptor’s sensitivity to extracellular calcium and lower PTH secretion.40-42 They also appear to have an effect on VDR by increasing its expression and reducing PTH43 synthesis.
In summary, the regulatory mechanisms of PTH are complex. Several factors are involved in this regulation and they exert their actions through specific receptors by direct and indirect mechanisms that modulate the expression of those receptors, which at the same time modifies the level of response of parathyroid glands.
Acknowledgements
This study has been supported by Fondo de Investigaciones Sanitarias (FIS 02/0688) (ISCIII-Retic-RD06, REDinREN, (16/06) and Fundación Renal Íñigo Álvarez de Toledo. During the development of this project, Natalia Carrillo-López received a grant from the Fundation Renal Íñigo Álvarez de Toledo, and later a research contract from REDinREN (ISCIIIRetic-RD06, REDinREN 16/06) Pablo Román-García and Dr. Daniel Álvarez-Hernández, Dr. Ignacio González-Suárez and Dr. ManuelNaves-Díaz also contributed to the development of the project.
Figure 1.
Figure 2.
Figure 3.