The most widely used approach in the immunotherapy treatment of cancer is the administration of monoclonal antibodies directed against regulatory molecules of immune control that inhibit the activation of T cells, the so-called check point inhibitors (ICI). ICI nephrotoxicity epidemiology and pathology; its diagnosis with or without kidney biopsy; the type and duration of treatment; the possibility of rechallenging after kidney damage; and its indication in patients with cancer and renal transplantation are certainly controversial. In the absence of definitive studies, this document is intended to specify some recommendations agreed by the group of Onconephrology experts of the Spanish Society of Nephrology in those areas related to ICI nephrotoxicity, in order to help decision-making in daily clinical practice in Onconephrology consultations.
El enfoque más utilizado en el tratamiento inmunoterápico del cáncer es la administración de anticuerpos monoclonales dirigidos contra moléculas reguladoras del control inmunitario que inhiben la activación de las células T, los llamados inhibidores del Check-Point (ICP). La epidemiología y patología de la nefrotoxicidad por los ICP; su diagnóstico con o sin biopsia renal; el tipo y la duración del tratamiento; la posibilidad de retratar después del daño renal; y su indicación en pacientes con cáncer y trasplante renal son ciertamente controvertidas. En ausencia de estudios definitivos, este documento está destinado a concretar unas recomendaciones consensuadas por el grupo de expertos de Onconefrología de la S.E.N en aquellas áreas relacionadas con la nefrotoxicidad por los ICP, con la finalidad de ayudar en la toma de decisiones en la práctica clínica diaria de las consultas de Onconefrología.
The Onconephrology group of the Spanish Society of Nephrology (SEN) was created with several purposes: to provide medical education; to carry out clinical, therapeutic and epidemiological studies; and to organize registries in the cancer-kidney area. An important objective was to clarify and provide recommendations about certain controversies on aspects that lack a precise information due to the absence of randomized and prospective studies.1 The renal damage associated with check-point inhibitors (CPIs), their diagnosis with or without the information of a renal biopsy, the type and duration of treatment, the possibility of restarting immunotherapy after the renal event and their indication in patients with cancer and renal transplantation are certainly controversial. The precise purpose of this article is, in the absence of solid scientific evidence, to make specify recommendations based on clinical experience and agreed upon by the SEN Onconephrology expert group when dealing with renal damage associated with the use of CPIs.
This document is not intended to represent a set of new guidelines, since it is not the result of a systematic review of the evidence, but is intended to be of useful to the nephrology specialist in the management of renal complications arising from treatment with CPIs in the daily clinical practice of onconephrology consultations.
Check-point inhibitors (CPIs)ICPs or immune checkpoint inhibitors2 are monoclonal antibodies directed against immune checkpoint regulatory molecules that inhibit T-cell activation. These drugs can prolong the survival of patients with different types of cancer such as melanoma, non-small cell lung cancer, urothelial cancer, renal cell cancer and many others.3
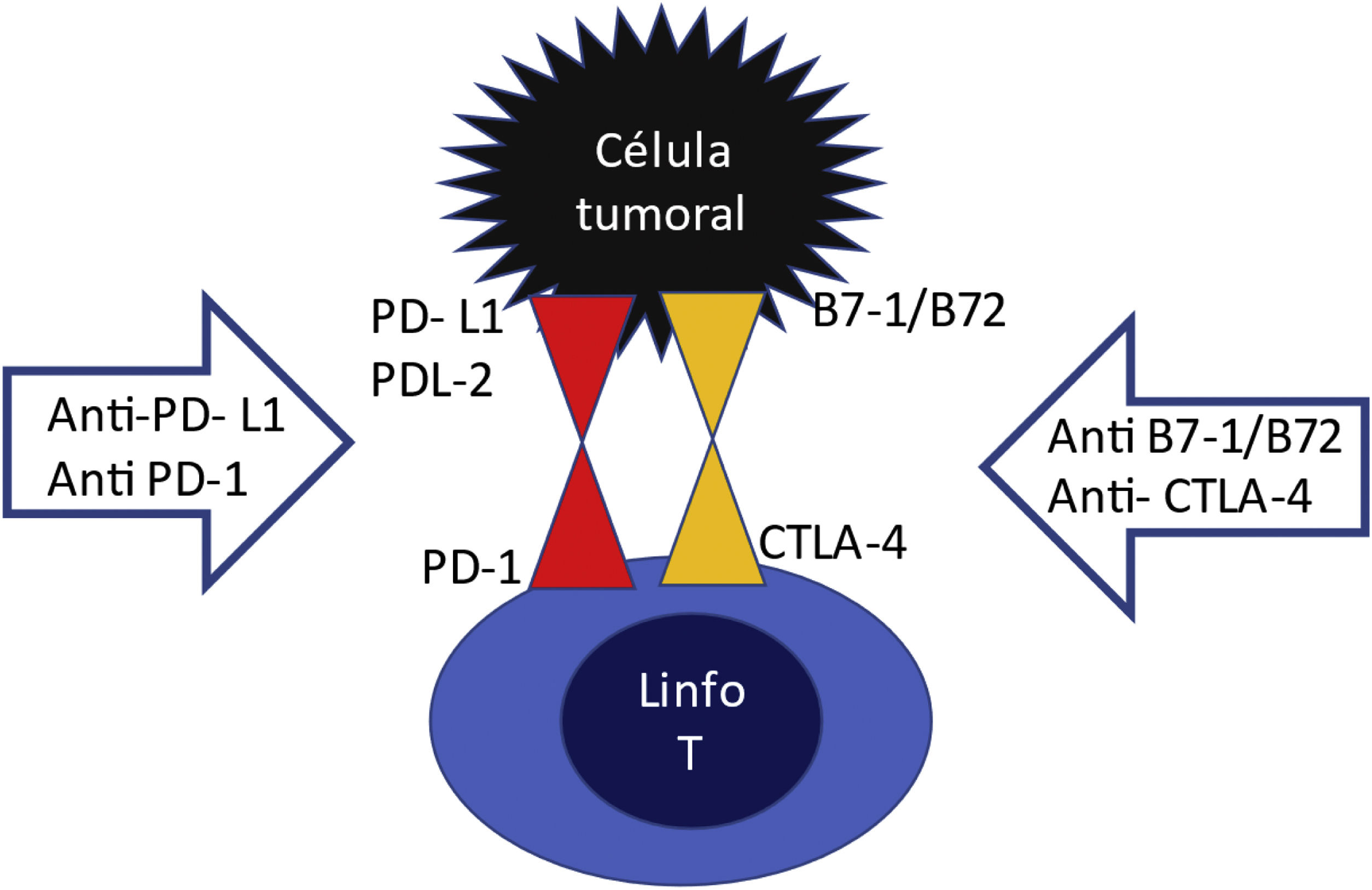
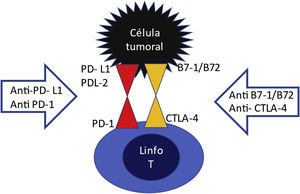
On the surface of T lymphocytes have there are inhibitory receptors PD1 (programmed death receptor) and CTLA4 (cytotoxic T lymphocyte associated antigen) which are like "handbrakes" that prevent an excessive T cell activation (Fig. 1). These inhibitory receptors are activated upon the binding to their ligand PDL1 or PDL2, or B7-1/B7-2, which are normally expressed on the surface of dendritic cells and macrophages. This effect is used by a multitude of tumors through the expression of PDL1 and PDL2 or B7-1/B7-2 ligands on their surface, thus escaping the action of the immune system. In other words, the tumor cell learns to activate the "hand brake". The ICPs are anti-PD1, anti-PDL1 and anti-CTLA4 agents. They block the lymphocyte receptor or block the tumor ligand, so that these two do not assemble and if they do not, the lymphocyte will continue to be activated to destroy the tumor, but nevertheless, by maintaining this overactivation of the T lymphocyte, these CPIs drugs can produce adverse effects related to the immune response, including renal damage.4
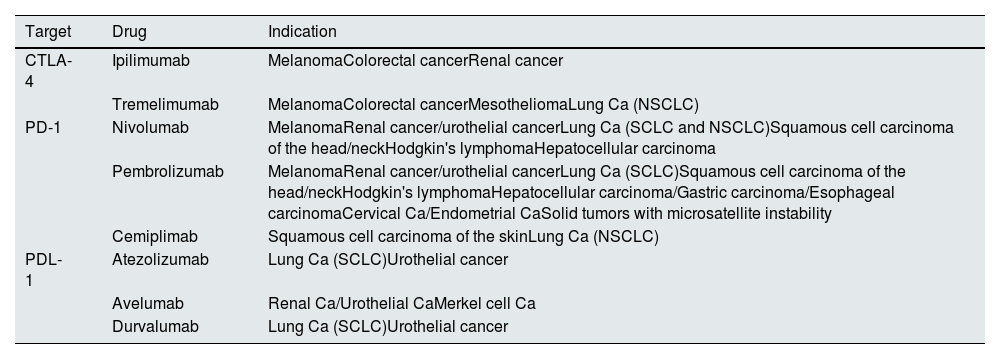
One CTLA-4 inhibitor, 3 antiPD-1 and 3 antiPD-L1 are approved for the treatment of various types of malignancies and their indications can be seen in Table 1.5 CPIs have long half-lives (6–27 days) and since they are eliminated mainly by proteolytic degradation within the liver, a decreased renal function does not affect its clearance.6
FDA- and EMA-approved CPIs.
| Target | Drug | Indication |
|---|---|---|
| CTLA-4 | Ipilimumab | MelanomaColorectal cancerRenal cancer |
| Tremelimumab | MelanomaColorectal cancerMesotheliomaLung Ca (NSCLC) | |
| PD-1 | Nivolumab | MelanomaRenal cancer/urothelial cancerLung Ca (SCLC and NSCLC)Squamous cell carcinoma of the head/neckHodgkin's lymphomaHepatocellular carcinoma |
| Pembrolizumab | MelanomaRenal cancer/urothelial cancerLung Ca (SCLC)Squamous cell carcinoma of the head/neckHodgkin's lymphomaHepatocellular carcinoma/Gastric carcinoma/Esophageal carcinomaCervical Ca/Endometrial CaSolid tumors with microsatellite instability | |
| Cemiplimab | Squamous cell carcinoma of the skinLung Ca (NSCLC) | |
| PDL-1 | Atezolizumab | Lung Ca (SCLC)Urothelial cancer |
| Avelumab | Renal Ca/Urothelial CaMerkel cell Ca | |
| Durvalumab | Lung Ca (SCLC)Urothelial cancer |
Ca: carcinoma; CPI: check-point inhibitors; NSCLC: non-small cell lung cancer; SCLC: small cell lung cancer; SCLC: small cell lung cancer; NSCLC: non-small cell lung cancer.
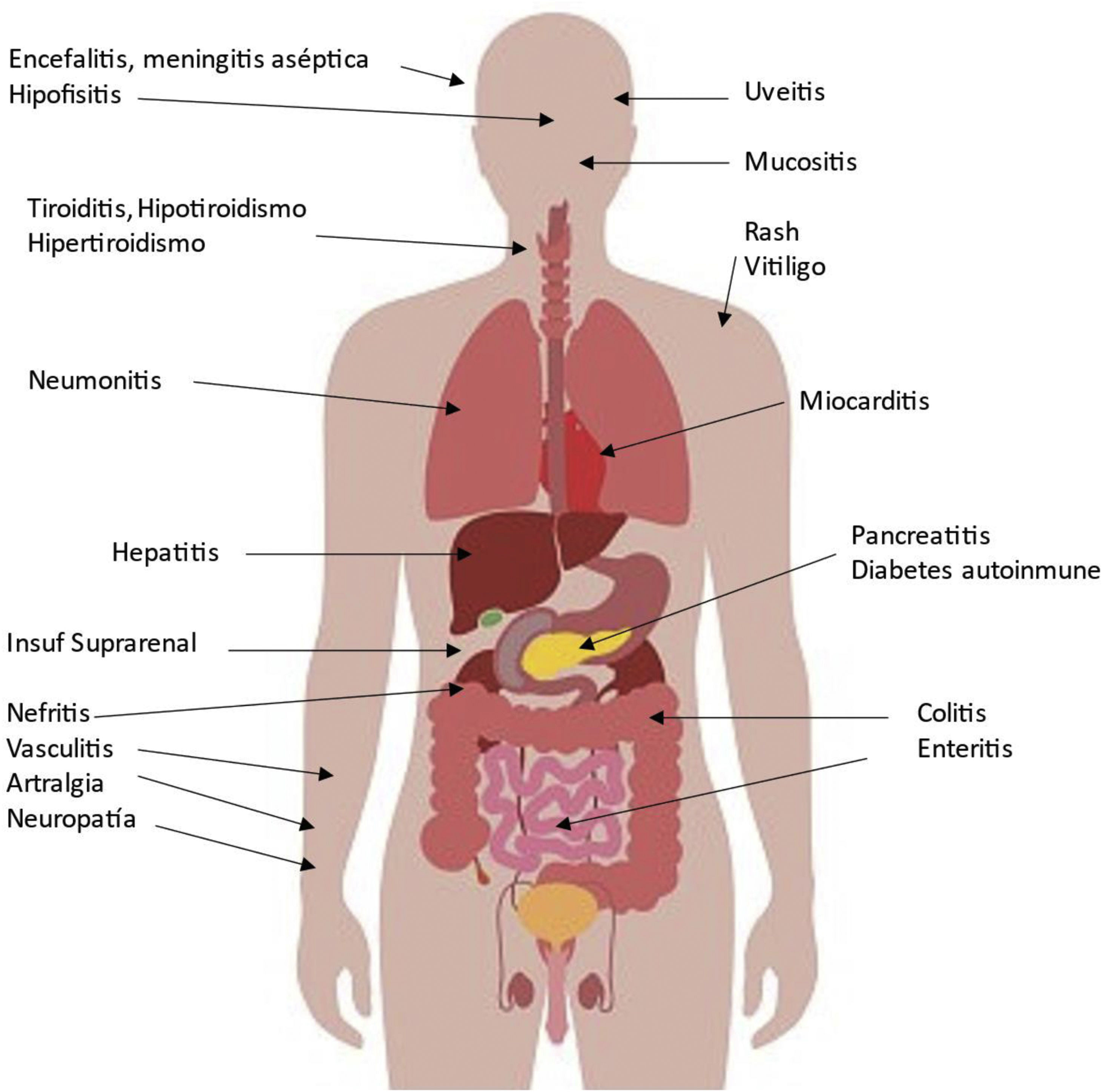
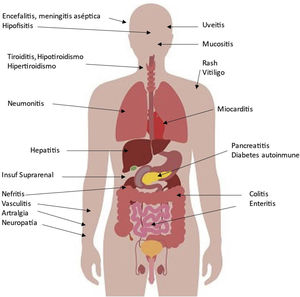
Possible side effects in various organs associated with CPIs are shown in Fig. 2. The organ damage is a consequence of increasing the activity of the immune system, which is especially important in patients with pre-existing autoimmune disease or a history of organ transplantation as we will comment. The incidence can be as high as 59–85%, depending on the agent, the use of a single agent or a combination of several CPIs. These adverse events usually develop within the first few weeks or months after initiation of treatment, but can occur at any time, even after cessation of CPI treatment, and diminish over time.7 Because of their eventual severity they require early detection and appropriate management. Although they can be controlled with corticosteroids and other immunosuppressants, major and even fatal secondary complications may occur in a small proportion of patients.
Mechanisms that may influence the occurrence of immune-related adverse effects following CPIs treatment include the following7:
- -
Increased T-cell activity against antigens that are present in tumors and healthy tissue.
- -
Increased levels of pre-existing autoantibodies (antithyroid antibodies, for example) or to drugs (haptens) that had been previously tolerated.
- -
Increase in the level of inflammatory cytokines.
- -
Complement-mediated inflammation due to direct binding of an anti-CLA 4 antibody to CTLA-4 occurring at the pituitary level.
The most common renal complication of the treatment with CPIs is acute renal failure. According to the available published series, the most frequent histological pattern of CPIs induced acute renal failure is acute immune-mediated tubulointerstitial nephritis (ITIN), which accounts for more than 90% of cases, however CPIs can also be associated with glomerular pathology. Cases of acute tubular necrosis (ATN) have been documented in patients on CPIs, although this injury does not seem to be pathophysiologically related to immunotherapy.
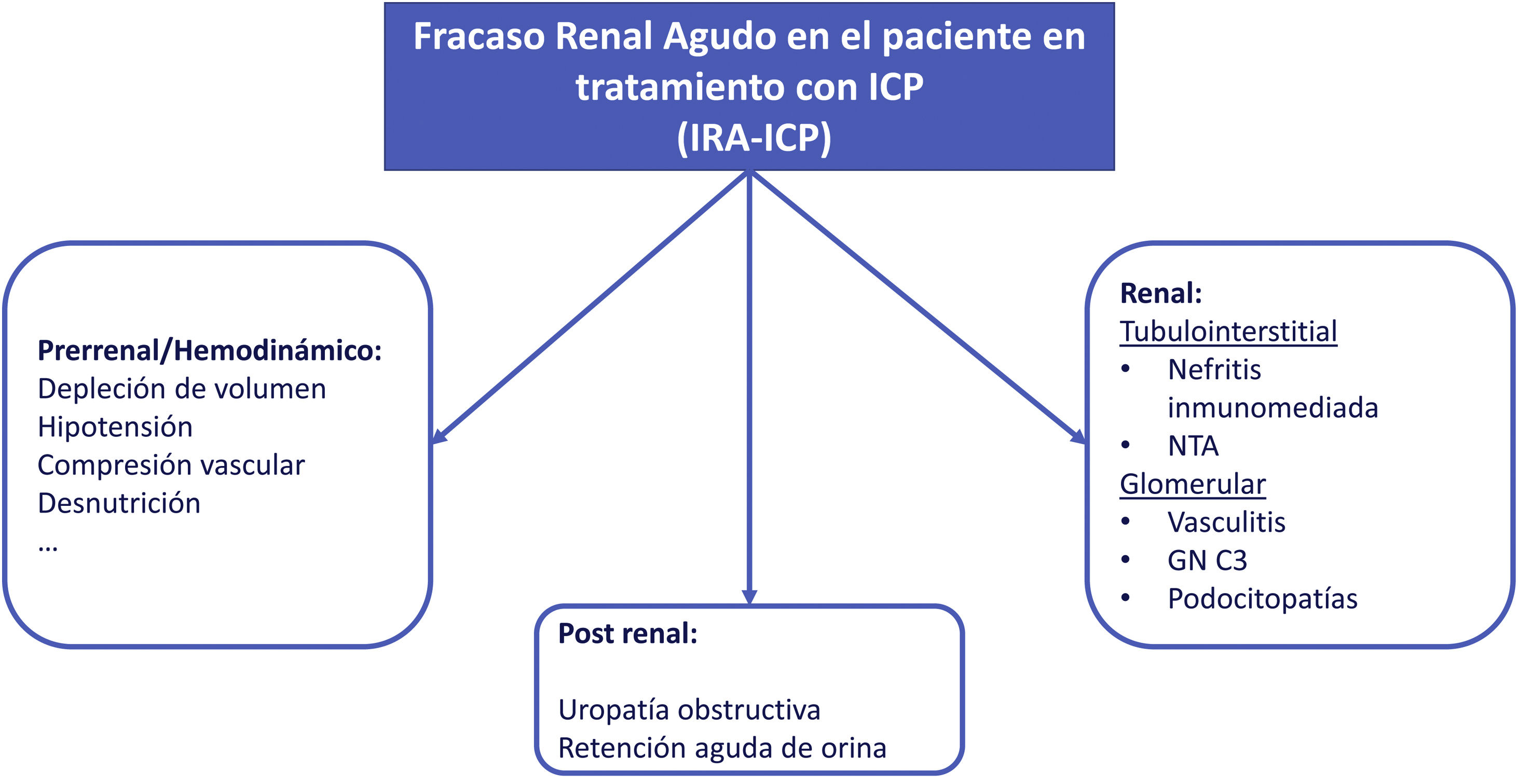
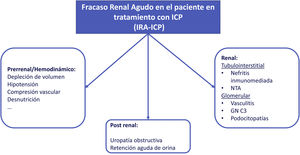
Acute renal failure (AKI)The etiology of AKI in patients undergoing CPI treatment may be multifactorial (Fig. 3). In one of the largest series published to date, less than 40% of AKI in patients under CPI treatment were directly associated with these drugs.8 If the AKI is attributed to oncospecific treatment it would require the withdrawal of the medication and initiation of corticosteroid treatment if NTIA is suspected; this strategy may lead to possible detriment to the evolution of the neoplasm. Therefore, assessment and exclusion of other etiologies is strongly recommended.
The incidence attributed to CPIs in the presence of AKI is estimated at 2−3%, with a mean onset of 15 weeks after initiating treatment.9–11 In general, the association of CTLA-4 and PD-1 inhibitors seems to result in a somewhat higher rate (5%).12
Risk factors for the development of CPI-AKI are: baseline renal function, the use of proton pump inhibitors (PPIs), the previous presence of other IRAES Is and the use of two or more CIPs, and patient's age.9–13
The PPIs along with other medications that cause ATIN, such as non-steroidal anti-inflammatory drugs (NSAIDs) or certain types of antibiotics, should be used with caution in patients receiving CPI and should be discontinued in those who develop CPI-associated acute renal failure (CPI-AKI).5 It has been shown that in those patients that develop CPI-AKI who were receiving medication that has been associated with ATIN, discontinuation of these treatments facilitates complete recovery of renal function.9 Given that the use of PPIs is a risk factor for the development of AKI in these patients, the authors of this article recommend avoiding their indiscriminate use, mainly in those patients with other additional risk factors.
Although the existence of a previous adverse immune effect (myocarditis, thyroiditis, colitis…) supports the possibility of CPI-AKI, only a 43% of patients with CPI-AKI have had this complication previously, so its absence does not rule it out.9
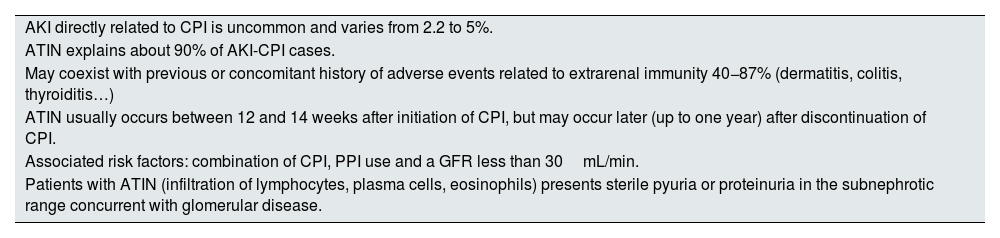
Acute tubulointerstitial nephritis (ATIN)Most characteristic features of ATIN associated with CPI are summarized in Table 2. ATIN is by far (>90%) the most frequent histopathological finding in renal biopsies of patients with CPI-AKI. Cortazar et al. in a multicenter study of 138 patients found ATIN as the dominant lesion in 93%9 of cases.
Most characteristic aspects of ATIN associated with CPI.
| AKI directly related to CPI is uncommon and varies from 2.2 to 5%. |
| ATIN explains about 90% of AKI-CPI cases. |
| May coexist with previous or concomitant history of adverse events related to extrarenal immunity 40−87% (dermatitis, colitis, thyroiditis…) |
| ATIN usually occurs between 12 and 14 weeks after initiation of CPI, but may occur later (up to one year) after discontinuation of CPI. |
| Associated risk factors: combination of CPI, PPI use and a GFR less than 30mL/min. |
| Patients with ATIN (infiltration of lymphocytes, plasma cells, eosinophils) presents sterile pyuria or proteinuria in the subnephrotic range concurrent with glomerular disease. |
PPIs: proton pump inhibitors; CPIs: check-point inhibitors; AKI-CPI-: acute renal failure associated to check-point inhibitors; AKI: acute renal failure; ATIN: acute tubulointerstitial nephritis.
Immune-mediated interstitial nephritis is caused either by loss of tolerance to renal autoantigens mediated by lymphocyte hyperactivation or by reactivation of exhausted T cells specific for certain drugs (PPIs, NSAIDs…) previously suppressed by PD1/CTLA4 signals.8,14,15
Immune-mediated nephritis has a different clinical presentation from the classic interstitial nephritis: Draibe et al. studied the differences between immuno-mediated AIN and classic ATIN associated with other drugs. They found a longer latency period since the introduction of the drug to the development of AKI, as well as a slower recovery from AKI in the group of patients who presented with immunotherapy-mediated ATIN.16 In other words, with the same histology, these appear to be two entities with different pathophysiological mechanisms.
Acute tubular necrosis (ATN)This is not a predominant lesion in AKI associated with CPI, but there are some publications that have observed this lesion more frequently than usual. Izzedine et al.17 studied 12 patients treated with pembrolizumab and the kidney biopsy showed that 4 patients had ATIN, while 5 had isolated acute tubular injury (ATN). In these cases, steroid treatment would not be indicated and probably neither would CPI withdrawal, which set up the discussion below about the need for a renal biopsy before the decision of corticosteroid treatment in patients with AKI and CPI treatment.
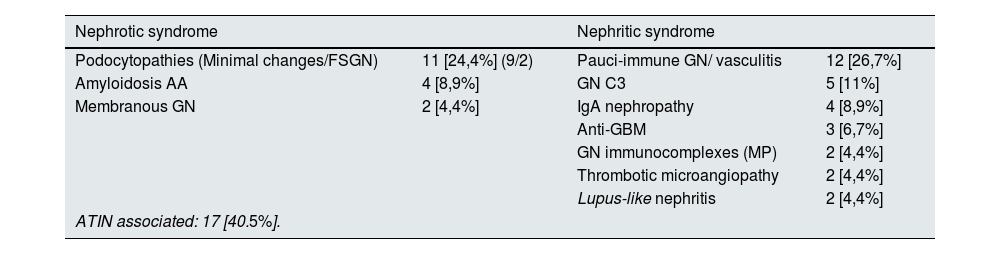
Glomerular injury associated with CPIThere have been described a number glomerular diseases associated with CPI treatment. Kitchlu et al.18 performed a systematic review and meta-analysis including 27 articles with 45 cases showing of glomerular disease with findings that clarify this pathology: paucimmune glomerulonephritis and renal vasculitis (27%); podocytopathies (minimal glomerular lesions in 9 and focal segmental hyalinosis in 2) (24%); and C3 glomerulopathy (11%). The results are summarized in Table 3. It is important to highlight that 41% of the cases had ATIN and that only 2 of the patients with vasculitis were ANCA positive. Mortality was high in this group despite treatment with corticosteroids. More than 60% of patients with podocytopathies had total or partial remission after drug withdrawal and corticosteroids and most C3 patients remitted with steroids alone. Therefore in some patients with glomerular damage the use of CPI could be resumed, particularly in those with podocytopathies although still we need more solid data to support such recommendation. Nevertheless, the evolution of the cases with glomerular disease induced "de novo" by CPI is not good. Most of the patients had their CPI interrupted (88%) and almost all of them received corticosteroid treatment (98%). Dialysis was required in 25%. Most patients had total (31%) or partially (42%) recovery from AKI; however, 19% remained dialysis-dependent, and approximately one-third died. Complete or partial remission of proteinuria was achieved in 45% and 38%, respectively.
Glomerular nephropathies associated with CPI.
| Nephrotic syndrome | Nephritic syndrome | ||
|---|---|---|---|
| Podocytopathies (Minimal changes/FSGN) | 11 [24,4%] (9/2) | Pauci-immune GN/ vasculitis | 12 [26,7%] |
| Amyloidosis AA | 4 [8,9%] | GN C3 | 5 [11%] |
| Membranous GN | 2 [4,4%] | IgA nephropathy | 4 [8,9%] |
| Anti-GBM | 3 [6,7%] | ||
| GN immunocomplexes (MP) | 2 [4,4%] | ||
| Thrombotic microangiopathy | 2 [4,4%] | ||
| Lupus-like nephritis | 2 [4,4%] | ||
| ATIN associated: 17 [40.5%]. | |||
AA, serum amyloid A; GN, glomerulonephritis; C3 GN, C3 glomerulonephritis; GBM, glomerular basement membrane; FSGN, focal segmental pattern glomerulopathy; CPI, check-point inhibitors; AITIN, acute tubulointerstitial nephritis.
The mechanisms by which CPIs induce glomerular disease are still poorly understood. Different hypotheses have been proposed, all of them had been already discussed previously in adverse events related to CPIs. An increase in inflammatory processes by T-cell hyperactivity, autoantibody production, or by inflammation related to C5a production that has been found to be increased when PDL-1 is blocked.19
When to indicate a renal biopsy in patients on treatment with CPI and renal damageThis discussion is based on the fact that since most of the renal lesions produced by CPI are ATIN, the risks of renal biopsy can be avoided if glomerular disease is not suspected. Initially patients can be treated with steroids and, depending on the response, decide the need to perform a biopsy. Many specialists believe that a clinical suspicion together with supporting laboratory data (e.g., eosinophilia, sterile pyuria, etc.) are sufficient to make a diagnosis.
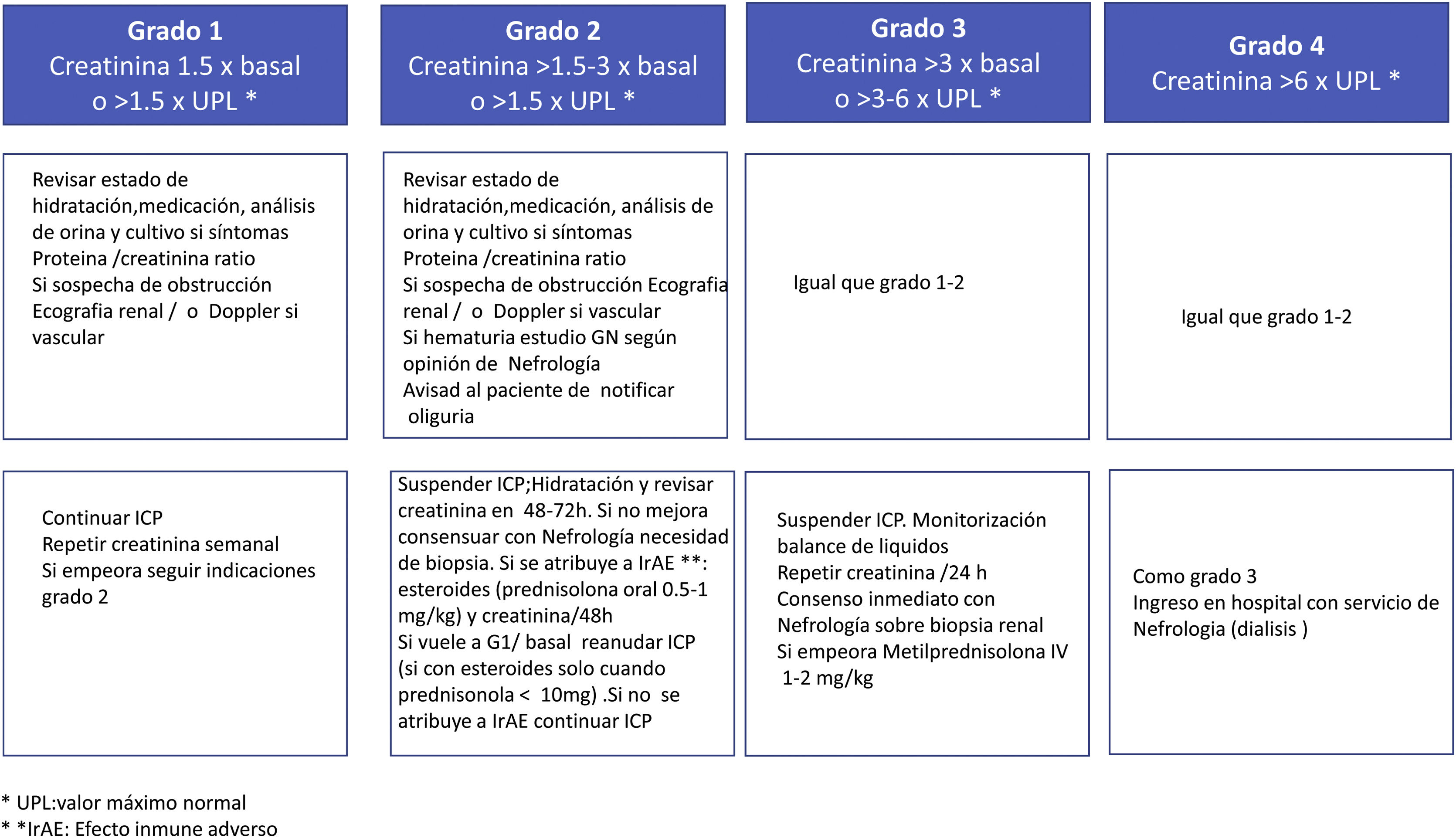
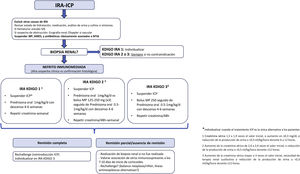
In fact, the American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) guidelines recommend that if there is no an alternative cause of AKI, physicians should proceed directly with immunosuppressive corticosteroid therapy without a renal biopsy specimen unless there is AKI with moderate/severe life-threatening potential (Fig. 4).20,21 In contrast, others believe that, in most patients, ATIN can only be diagnosed with a renal biopsy as many of these clinical data are absent in biopsied patients and only 50% present with extrarenal manifestations.6 The rationale for or against a renal biopsy in patients with AKI treated with CPI are some of the following22:
In favor of renal biopsy
▪Cancer patients treated with CPI who develop AKI may have ATN (a common cause of AKI in oncology patients) or other renal lesions instead of ATIN.
▪Identification of renal lesions that are not ATIN, such as ATN, avoids the use of corticosteroid and makes possible to maintain CPI therapy. The assumption that patients have ATIN may expose them to unnecessary corticosteroids (with their associated complications) and the unwise decision of discontinuation of CPI therapy. Ultimately, this approach may lead to substandard outcomes of cancer treatment.
▪Histological confirmation of the type of renal lesion is essential in order to be able to reintroduce immunotherapy if necessary. Assuming an ATIN may imply the definitive suspension of CPI, especially in cases of AKI grade >1 and in those patients receiving the drug within a clinical trial.
▪The histologic pattern observed will guide the treatment (use of other immunosuppressors in glomerular pathology).
▪Quantification of interstitial fibrosis allows to be made a renal prognosis.
Against renal biopsy
▪The form of AKI most frequently associated with the use of CPI is ATIN, so treatment with corticosteroids could be assumed.
▪Renal biopsy is a procedure with possible serious complications.
▪Complete or incomplete remission can be achieved with corticosteroid treatment in a high percentage of cases.
The group has specified its position regarding the obtention of renal biopsy in patients with AKI-CPI. The recommendations are based on its experience and on different publications5,6,9,10,13,17,18,20–22 and considers that renal biopsy is a fundamental tool for the management of these patients, since it allows confirmation of the diagnosis (avoiding treatment with corticosteroids or the withdrawal of CPI in cases in which the biopsy does not confirm the presence of AIN) and helps to establish the prognosis according to the degree of fibrosis.
It is important to remember that oncology patients often have other risk factors for renal damage (hypoperfusion episodes, iodinated contrast agents, tubular toxic agents such as platinum) and that AKI recovery in non-biopsied, corticosteroid-treated patients may reflect the natural course of ATN recovery and have nothing to do with corticosteroid treatment, which makes the argument that renal recovery in patients receiving corticosteroids is confusing. Many oncologists consider that an empiric course of corticosteroids is a strategy that carries low risk in these patients, and currently recommend this approach in their guidelines.20 One could argue that this approach is reasonable in patients who have other immune-mediated organ injury, avoiding the potential complications of renal biopsy. We agree with Gupta et al.5 that empirical steroid therapy should only be considered for patients with the absence of a plausible alternative etiology for AKI and in those with an absolute contraindication to renal biopsy due to safety concerns (e.g., uncontrolled hypertension or anticoagulation). The presence of a single kidney (e.g., due to nephrectomy in renal cancer) is not an absolute contraindication for biopsy, although of course, the risk-benefit should be assessed carefully. Knowing the type of renal lesion is important because corticosteroids and/or drug withdrawal may be necessary for patients with ATIN, but are not required for ATN and other non-immune-mediated lesions.
Thus, we consider that unless absolutely contraindication, renal biopsy should be performed in all patients to obtain the diagnosis of the renal damage associated with CPI, and preferably before 48−72h. In certain circumstances with mild AKI in which the response to steroids is excellent and the clinical suspicion of immune-mediated nephritis is very high, biopsy we can avoided and consider it if there is deterioration of renal function after rapid tapering/withdrawal of steroids.
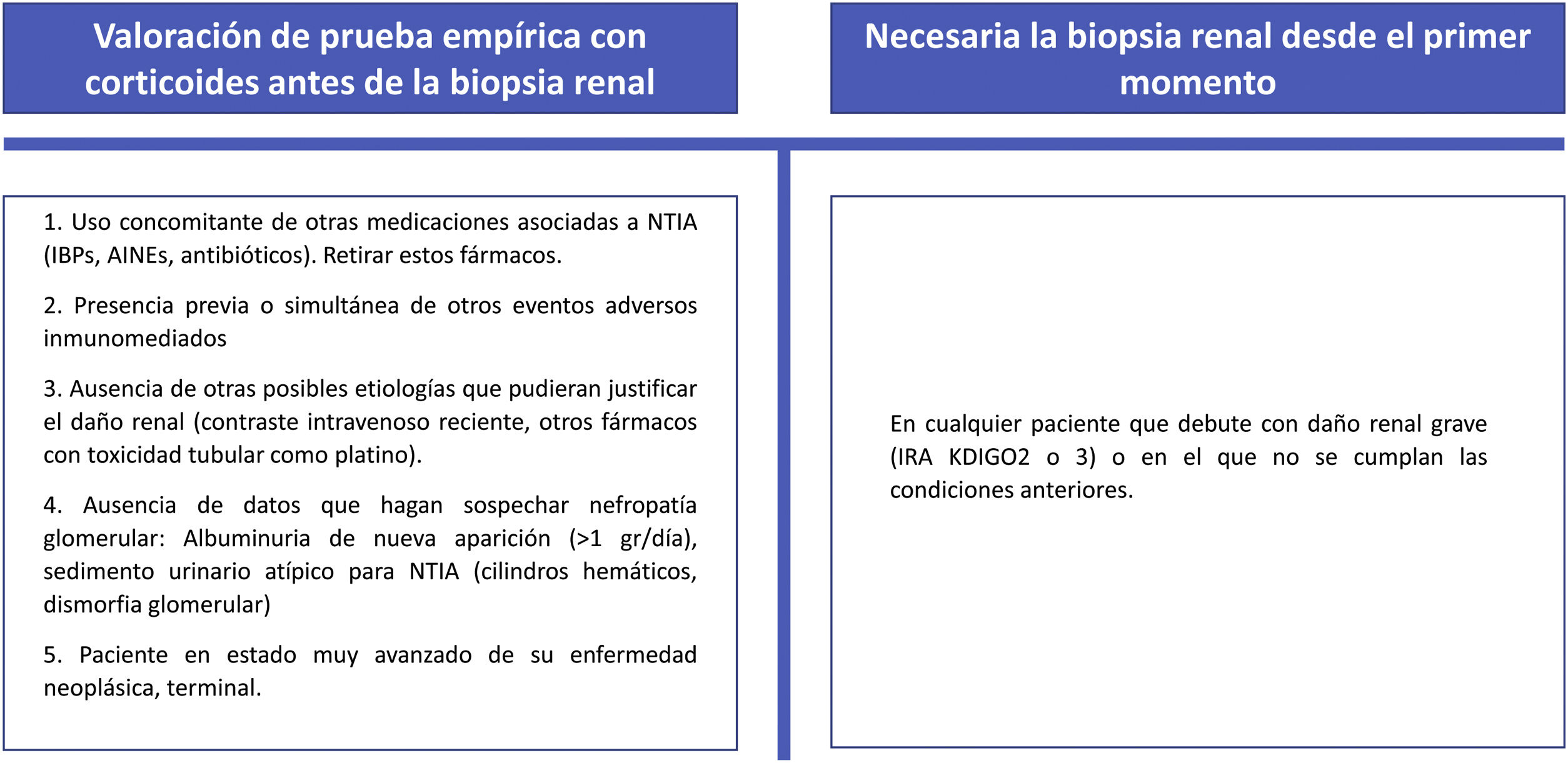
Circumstances that might justify a short empirical test with corticosteroids prior to biopsy and those in which we consider a renal biopsy to be necessary from the outset are shown in Fig. 5.
If the renal biopsy is delayed due to patient characteristics or to the management of the hospital service and the suspicion of immune-mediated nephritis is high, the initiation of corticosteroids should not be delayed until the biopsy is performed.
The authors propose the following indications for renal biopsy in patients with renal damage associated with CPI- 1
Patient presenting persistent AKI grade 1 or significant AKI grade ≥2 before initiating corticosteroid therapy.
- 2
Absence of contraindications (e.g., risk of bleeding, uncontrolled HT, severe thrombocytopenia).
- 3
Absence of other possible etiologies (recent intravenous contrast, medications, optimization of hydration status).
- 4
Absence of medication that produces ATIN (PPIs, NSAIDs, antibiotics).
- 5
Absence of other adverse immune effects requiring steroid treatment (postpone renal biopsy if patient is on steroids and check serum creatinine).
- 6
Suspected glomerular nephropathy: new-onset albuminuria (> 1g/day), urinary sediment atypical for ATIN (hematic casts, glomerular dysmorphia), nephrotic syndrome, rapidly progressive renal failure.
- 7
Concurrent nephrotoxic chemotherapeutic agents.
- 8
Non-response to the usual corticosteroid therapy indicated in ATIN.
Once other causes of AKI have been excluded, and the diagnosis has been made, the management of ATIN includes evaluating the suspension of CPI treatment and treatment with corticosteroids, if there is no contraindication. The aim is to find a balance between preventing renal failure and keeping the progression of the cancer under control.
Corticosteroids are the mainstay of treatment of ATIN in patients receiving CPI. This is supported by observational studies since there are not randomized studies available. In the study by Cortázar et al.9 most patients were treated with corticosteroids (86%) and 45% had complete or partial recovery, respectively. Gupta et al.10 in a multicenter study of 429 patients with AKI associated with CPI treatment found that renal recovery occurs in approximately two-thirds of patients, and that early initiation of corticosteroids is associated with a higher likelihood of renal recovery. Other studies also report a 85% partial or complete recovery of renal function.11,23,24 Early treatment with corticosteroids is essential. In the multicenter study by Gupta et al.10 early initiation of corticosteroids (within 3 days of AKI-CPI) was associated with a higher likelihood of renal recovery compared to late initiation (more than 3 days after AKI-CPI). The CPIs have a long live, the period of treatment required to maintain the therapeutic response is unknown since there is currently an absence of randomized clinical trials in this regard. The same consortium led by Gupta et al. analyzed the steroid regimens of 165 patients with AKI associated with CPI in a retrospective study, concluding that a short steroid regimen (<28 days) in these patients presents similar results to a long regimen (29–84 days) in terms of recovery of renal function.25
Other immunosuppressive agents have also been investigated in the setting of AKI-CPI. Cortázar et al.9 used mycophenolate mofetil in 7 patients and obtained complete renal recovery in one patient, with partial recovery in the remaining 6. Other agents that have been studied include rituximab, cyclophosphamide and infliximab, but these are exceptional cases that did not respond to corticosteroids.26
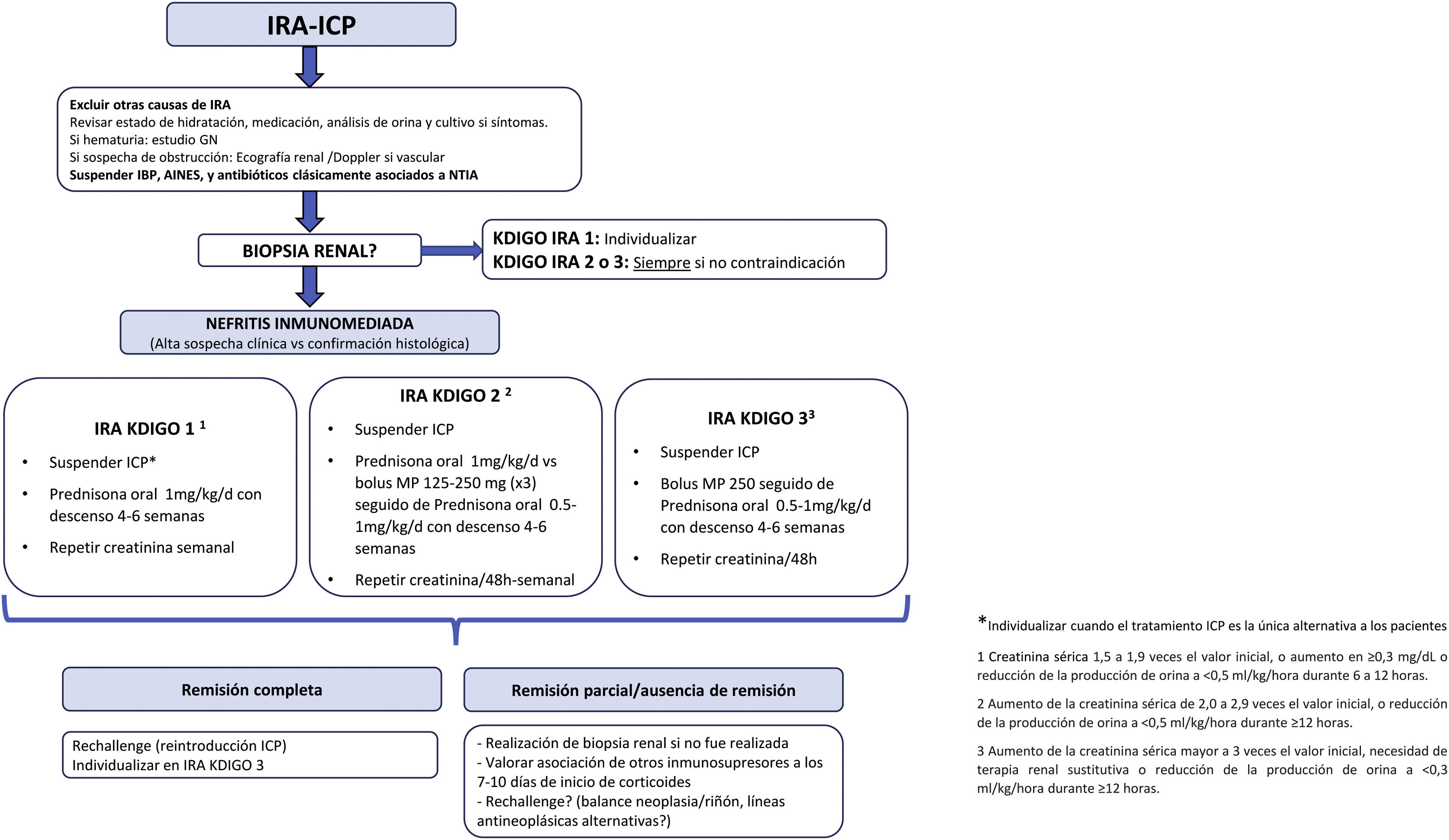
Positioning of the SEN Onconephrology Group on the treatment of patients with AKI-CPIThe treatment recommendations by the SEN Onconephrology group are as follows (Fig. 6).
Immunotherapy should be stopped for at least one or two cycles to verify that the corticosteroids are effective. There are exceptional cases when CPI treatment is the only alternative for patients, in which co-treatment with immunosuppression and CPI should be considered in patients with grade 1 AKI and the dilemma is cancer or kidney. Such a personalized treatment scheme is mainly the opinion of experts based on recommendations and there is currently little evidence to support it.
In patients with ARF grade 2−3 it is necessary to suspend treatment with CPI at least until the response to steroids is evaluated. In case of rapid improvement of renal function and absence of other serious side effects of immunotherapy, treatment could be continued.
The group's recommendations regarding steroid therapy- 1
Rule out other causes of AKI. In the case of new onset proteinuria (>1g/day) and/or urinary sediment atypical for ATIN a renal biopsy should be performed.
- 2
Discontinue PPIs, NSAIDs, and antibiotics associated with ATIN.
- 3
The mainstay of treatment in ATIN associated with CPI is glucocorticoids (GC), although scientific evidence is still scarce. Two types of treatment are recommended, which we summarize below:
GCs are used as the initial IV treatment to maximize exposure at the start of treatment and reduce the adverse effects of prolonged administration of high-dose. We propose treatment with bolus methylprednisolone 125−250mg for 3 days and continuation with prednisone at 0.5−1mg/kg/d if the patient has presented with AKI-CPI KDIGO 3 or KDIGO 2.
The descending corticosteroid regimen will be carried out during a period of 4–6 weeks (in some cases it could be considered up to 8 weeks), with the following schedule:
Oral prednisone
Week 0 to 1: 30−40mg*
Week 1 to 2: 20−30mg.
Week 2 to 3: 10−20mg.
Week 3 to 4: 5−10mg.
Week 4 to 5: 0−5mg.
Week 5 to 6: discontinue
* 0.5mg/kg/day with a maximum of 40mg/d starting and continue according to the schedule presented above
Protocol B: oralThere should be a close analytical reevaluation. We propose treatment with prednisone at 1mg/kg/d if the patient has presented AKI-PKD KDIGO 1 or 2, with the following scheme:
Week 0 to 1: 60−80mg*
Week 1 to 2: 40−60mg.
Week 2 to 3: 20−40mg.
Week 3 to 4: 10−20mg.
Week 4 to 5: 5−10mg.
Week 5 to 6: discontinue
*1mg/kg/d with a maximum of 80mg/d to start and continue according to the attached guideline.
The benefit of adding another immunosuppressant (MMF or infliximab) is to reduce dose of steroids in the patient who responds partially or presents intolerance and/or associated side effects, or relapses after reducing steroids. These treatments must be agreed upon, they should never be administered without prior consensus with Oncology since they can have harmful effects on the evolution of the neoplastic pathology.
Resuming the treatment after adverse effects with check-point inhibitors (Rechallenge)Since most immune-related adverse events resolve within weeks or months after treatment with immunosuppressants (usually corticosteroids), one of the most important issues in clinical practice is the safety of resuming treatment after the adverse event has resolved.
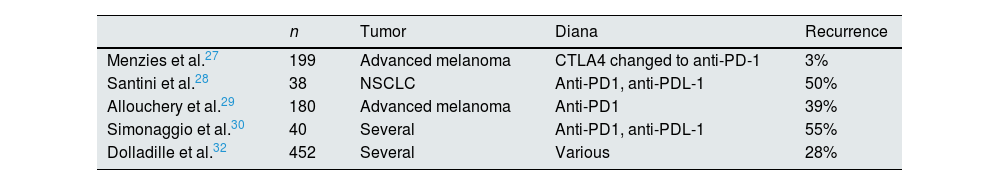
Resuming the treatment after non-renal adverse immunologic effects-CPI: recurrence of effectsThere are only retrospective observational studies analyzing the incidence of overall adverse immune effects upon resumption of treatment; results were different depending on the drugs and their substitution; recurrent or new adverse effects may be observed. A large pharmacovigilance cohort study in individual cases from the WHO database evaluates the recurrence rate of immune adverse effects in patients after re-starting CPI treatment and found a 29% of adverse effect recurrences, most frequently colitis, hepatitis and pneumonitis27–32 (Table 4).
Resuming CPI after non-renal adverse immunological effects-CPI: recurrence of adverse effects.
| n | Tumor | Diana | Recurrence | |
|---|---|---|---|---|
| Menzies et al.27 | 199 | Advanced melanoma | CTLA4 changed to anti-PD-1 | 3% |
| Santini et al.28 | 38 | NSCLC | Anti-PD1, anti-PDL-1 | 50% |
| Allouchery et al.29 | 180 | Advanced melanoma | Anti-PD1 | 39% |
| Simonaggio et al.30 | 40 | Several | Anti-PD1, anti-PDL-1 | 55% |
| Dolladille et al.32 | 452 | Several | Various | 28% |
CPI: check-point inhibitors; NSCLC: non-small cell lung cancer.
When a patient has had an episode of AKI-CPI, especially grade 2−3, and such treatment is necessary for cancer remission, the decision to resume CPI is complex because it could lead to new episodes of AKI which worsens the prognosis severely. According to the ASCO guidelines (Fig. 4), CPI treatment should be permanently discontinued in all patients who develop grade 2 AKI if it does not improve within days with steroids and in grade 3.20 The problem with this view is that this approach may deprive patients of potentially life-saving therapy.
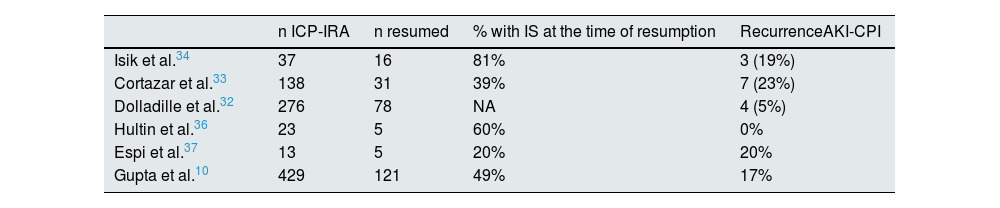
The recurrence of AKI-CPI after retreatment has been evaluated in several studies and the results have been variable, but in general it is estimated to be around 20% and most of them were still receiving steroids at the time of re-exposure10,32–37 (Table 5). Given the low incidence of recurrent AKI-CPI, it seems reasonable to consider re-exposure in patients for whom CPI is the optimal treatment,27 probably with concomitant low-dose steroid therapy.
Resuming CPI after AKI-CPI: recurrence of AKI.
| n ICP-IRA | n resumed | % with IS at the time of resumption | RecurrenceAKI-CPI | |
|---|---|---|---|---|
| Isik et al.34 | 37 | 16 | 81% | 3 (19%) |
| Cortazar et al.33 | 138 | 31 | 39% | 7 (23%) |
| Dolladille et al.32 | 276 | 78 | NA | 4 (5%) |
| Hultin et al.36 | 23 | 5 | 60% | 0% |
| Espi et al.37 | 13 | 5 | 20% | 20% |
| Gupta et al.10 | 429 | 121 | 49% | 17% |
AKI-CPI: acute renal failure associated with check-point inhibitors.
The decision to restart CPI treatment depends on the severity of the previous event and the degree of recovery from such event, the availability of alternative therapeutic options, and the overall status of the cancer. There are three important aspects to consider. First, the severity of the adverse event, because if there is life-threatening toxicity, particularly cardiac or neurological toxicity, or a massive release of cytokine, CPI should not be restarted. Also need to consider the presence of drugs that can produce ATIN-AKI and that can be withdrawn (PPIs, NSAIDs…) and the combination of CPI treatments. With these considerations and after immunosuppressive treatment (preferably corticosteroids) had produced complete or partial remission, Hermann35 proposes several possibilities:
- 1
Change CPI class if deemed appropriate and plausible by Oncology. For example, the incidence of ICP-AKI is less than 1% with PDL1 blockade compared to an incidence of 2–5 % with other classes.38
- 2
Continue with the same CPI or in case of double therapy suppress CTLA-4 and continue with anti-PD-1 monotherapy.
- 3
Secondary prevention in which CPI is resumed concomitantly with immunosuppressive therapy: after the initial episode of CPI-AKI, immunotherapy is resumed upon resolution of the AKI, concomitantly with low doses of steroids (usually 10mg daily) depending on other factors such as severity of AKI and other concomitant adverse effects. However, if at resumption of CPI treatment the patient is no longer on steroids there is no evidence to recommend restarting steroids. This secondary prevention can be maintained for several cycles or continuously, but there is no published experience.
Therefore, oncologists, nephrologists and multidisciplinary teams have to balance the clinical benefit and the danger of treatment-related toxicities for each patient.39
Positioning of the SEN Onconephrology group on re-treatment (Rechallenge) with AKI-CPIRetreatment with CPI may be considered in patients with AKI-CPI.
- 1
If the cancer has not yet responded due to the short time of treatment or inadequate response to treatment
- 2
If the time period of resolution with corticosteroids of AKI-CPI is short, with creatinine decrease at 1 week (>25%).
- 3
If the patient is on treatments that may induce ATIN (NSAIDs, PPIs, antibiotics) that have been withdrawn at the diagnosis of nephritis.
- 4
In those receiving a combination of treatments that can be reduced to one.
- 5
Once renal response is achieved, it is advisable to reintroduce CPI when the patient is on a dose of prednisone <20−10mg/day and is continued with the tapering of steroid regimen.
The renal risk of reinitiating CPI should always be explained to the patient and balanced against the risk of progression of the neoplasm. Before making therapeutic decisions, it is advisable to consider whether the patient can undergo renal replacement therapy (RRT) by dialysis in case of poor evolution, taking into account the overall prognosis.
Retreatment with CPI should not be considered in patients with AKI-CPI.
- 1
History of serious life-threatening adverse immunological effects: neurological, pulmonary, myocarditis, cytokine release syndrome.
- 2
Availability of alternative treatments in patient on a critcal situation.
- 3
If the time to resolution of AKI-CPI with corticosteroids is slow with a decrease in creatinine at one week (<25%) (depending on the type of tumor) or poor renal recovery, leaving the patient with reduced "residual" renal function. If treatment with CPI is unavoidable due to the patient's oncologic situation, RRT should be prioritized if indicated but in agreement with the patient and then immunotherapy should be restarted.
- 4
In case of suspected glomerular nephropathy: Do not retreat without biopsy in those patients with new-onset proteinuria (>1g/day) and a urine sediment that is not compatible with ATIN.
- 5
Previous favorable response of the tumor treatment. If the target was achieved, it is reasonable to assume that the response is durable and retreatment should not be considered.
Cancer is one of the major causes of mortality in the solid organ transplant population.40 Immunosuppression may predispose to cancer by inhibiting immune surveillance, suppressing DNA repair and predisposing to oncogenic viral infections. The number of patients living with a transplanted kidney is increasing, so the indication for new immunotherapeutic treatments will also increase progressively.
Factors to be considered when CPI is indicated in renal transplant patientsNew treatments such as CPIs result in cellular and humoral graft rejection in 30−40% of which up to 60% result in kidney loss.41 There are several factors to be considered when using CPIs in kidney transplant recipients. Observational studies have shown that the use different CPIs produce diverse effects on acute rejection however further additional studies are needed.42–44
In a review of published cases and case series Gupta et al.5 found that the rejection rate with anti-cTLA-4 (ipilimumab) monotherapy was 33% as compared with 52% in patients receiving anti-PD-1 monotherapy. It is difficult to draw a definitive conclusion because patients had undergone different reductions or changes in immunosuppression at the time of diagnosis of metastatic cancer, which could strongly influence rejection rates. Therefore, further studies are needed.
It has been shown that increased immunosuppression at the initiation of CPI or the use of mTOR inhibitors was associated with a lower risk of rejection.41 Factors such as duration of transplantation, history of donor-specific antibodies or a previous history of rejection are also important risks to be considered.
A recent multicenter observational study by Murakami et al.41 examined the safety and efficacy of CPI in 69 kidney transplant patients from 23 institutions. The study showed that 42% of patients experienced acute allograft rejection with a median occurrence of 24 days since the initiation of CPI to rejection. Once rejection occurred, 65% of patients experienced allograft failure and required dialysis. A recent multicenter single-arm study analyzed 17 patients with kidney transplant aged >18 years treated with nivolumab for incurable, locally advanced cancer or metastatic solid tumors with a creatinine level <180mmol/L, and either without or low concentrations of donor-specific HLA antibodies. Baseline immunosuppression was not modified which did not affect the efficacy of CPI (similar to that described in this population), and it also resulted in lower rejection rates (12%) than previously published. There were no treatment-related deaths or serious adverse events.45
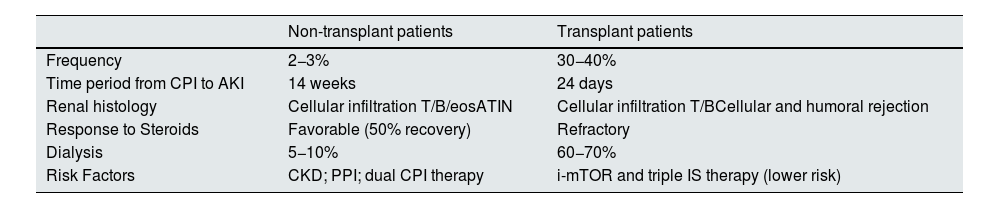
Table 6 shows the differential characteristics of AKI between non-transplanted patients (generally ATIN) and transplanted patients (cellular and humoral rejection). In transplants patients the AKI is much more frequent, appears earlier, is resistant to steroids and there is a high percentage of graft loss.46
Clinical characteristics of AKI associated with CPI treatment.
| Non-transplant patients | Transplant patients | |
|---|---|---|
| Frequency | 2−3% | 30−40% |
| Time period from CPI to AKI | 14 weeks | 24 days |
| Renal histology | Cellular infiltration T/B/eosATIN | Cellular infiltration T/BCellular and humoral rejection |
| Response to Steroids | Favorable (50% recovery) | Refractory |
| Dialysis | 5−10% | 60−70% |
| Risk Factors | CKD; PPI; dual CPI therapy | i-mTOR and triple IS therapy (lower risk) |
eos, eosinophils; CKD, chronic kidney disease; AKI, acute renal failure; PPI, proton pump inhibitors; AKI, acute renal failure; ATIN, acute tubulointerstitial nephritis; CPI, check-point inhibitors.
Blockade of CTLA-4 and PD-1 increases T-cell activation, not only against malignant cells, but also against other cells expressing foreign antigens, such as renal allograft donor antigens. PD-1 and PD-L1 can be found in the allograft and its reactive T cells43. The activation of T-cells may lead to acute cellular rejection and activated CD4 T cells could lead to B-cell proliferation and activation through co-stimulatory ligands (e.g., CD40L) and cytokines (e.g., IL-4, IL-21 and interferon-γ) leading to antibody-mediated rejection, especially if immunosuppression is reduced.47 B cells may also be activated as a direct effect on PD1-expressing memory B cells if there was prior sensitization of the transplanted organ or a decrease in immunosuppressive drugs.44
Preventive strategies for rejection control in transplant patients with cancer undergoing CPI treatmentSo far there are very few published cases, but it appears that PD-1 inhibitors may be more likely to cause rejection in the transplanted kidney than CTLA-4 antagonists. This is especially true when patients receive anti-CTLA-4 agents prior to treatment with PD-1 inhibitors.48
There is contradiction in the effect that corticosteroids may have on the antitumor response of CPIs in these cases. In some trials, it may have decreased the antitumor response of CPIs,49 while in others overall survival and time to treatment failure were not affected by the use of systemic glucocorticoids.50
Barnett et al.48 published the case of a kidney transplant patient with previous bilateral nephrectomy for renal cancer and a diagnosis of metastatic adenocarcinoma in treatment with nivolumab. He received a tapering steroid regimen that was initiated one week before the introduction of CPI, and tacrolimus was replaced by sirolimus with favorable cancer progression and stable renal function. The use of mTOR inhibitors (everolimus, temsirolimus and sirolimus) have been studied in many types of cancer and in these patient, sirolimus may have played a synergistic antitumor role in addition to being an immunosuppressive agent. Jhaveri F. refers a dozen patients with this therapeutic approach with good evolution.46
However, mTOR inhibitors are associated with a higher risk of rejection than anticalcineurin inhibitors. Depending on the immunologic risk between the donor organ and the recipient, the decision to reduce or discontinue immunosuppression must be balanced against the increased risk of rejection with CPI. More studies are needed, and actually there are ongoing several clinical trials, some of them to evaluate the potential effect of prophylactic steroid minipulses with CPI cycles and conversion of CNI to mTOR inhibitors to determine both rejection rate and cancer response.
Positioning of the SEN Onconephrology Group on the management of renal transplant patients with cancer receiving treatment with CPI- 1
The decision to initiate CPI treatment in kidney transplant patients is complex and it should be multidisciplinary, and always provide a correct information to the patient about the risks and benefits.
- 2
The following should be considered in the decision: the duration of the transplant, history of specific donor antibodies, previous history of rejection or the type of immunotherapy to be used (higher risk of rejection in anti-PD1 and anti-PDL1).
- 3
Decide against treatment with immunotherapy if an alternative treatment is available.
- 4
Consider increasing or restarting corticosteroids prior to treatment and assess the suitability of mTOR inhibitors.
None.
Conflict of interestM.J. Soler reports receiving lecture and scientific consulting fees from Astra Zeneca, Novo Nordsik, Esteve, Vifor, Bayer, Mundipharma, Ingelheim Lilly, Jansen, Fresenius, ICU Medical, Travere Therapeutics, and Boehringer.
The rest of the authors declare no conflict of interest for this work.