Acute kidney injury (AKI) is a serious pathology that progress with dysfunction of regulating blood pressure and fluid balance, concentrating urine due to decrement of aquaporin-1 (AQP) levels during the inflammation process. Irbesartan (IRN), angiotensin receptor blocker, is widely used in the treatment of hypertension, which also has anti-inflammatory, antioxidant and anti-apoptotic properties. The aim of this study is to investigate the protective effects of IRN in lipopolysaccharide (LPS)-induced kidney injury.
Material and methodsTwenty-four rats divided into three groups as control, LPS and LPS+IRN group. After 6h of LPS administration, rats were sacrificed. Blood samples and half of the kidney tissues were collected for biochemical analysis and remaining tissues were taken for histopathological and immunohistochemical analysis.
ResultsIn the LPS group, glomerular congestion and shrinkage, degeneration of distal tubules, mononuclear cell infiltration, cellular debris and intense proteinous accumulation in the tubules, increased expressions of Cas-3, nuclear factor kappa beta-p65 (NF-kB p65), levels of creatinin, TOS, OSI and decreased levels of TAS, AQP-1 were found significantly. IRN treatment reversed all these parameters. IRN's restorated AQP-1 levels by its anti-inflammatory, antioxidant and anti-apoptotic effects due to inhibiting NF-kB expression.
ConclusionThis study suggests that IRN can be used in conditions affecting the kidneys such as AKI. Further studies needed for detailed molecular investigation of IRN at different doses and durations.
La lesión renal aguda (IRA) es una patología grave que cursa con disfunción en la regulación de la presión arterial y el equilibrio hídrico, concentrando la orina debido a la disminución de los niveles de acuaporina-1 (AQP) durante el proceso inflamatorio. Irbesartan (IRN), bloqueador de los receptores de angiotensina, es ampliamente utilizado en el tratamiento de la hipertensión, que también tiene propiedades antiinflamatorias, antioxidantes y antiapoptóticas. El objetivo de este estudio es investigar los efectos protectores de IRN en la lesión renal inducida por lipopolisacáridos (LPS).
Material y MétodosVeinticuatro ratas divididas en 3 grupos como control, grupo LPS y LPS+IRN. Después de 6 horas de administración de LPS, se sacrificaron las ratas. Se recogieron muestras de sangre y la mitad de los tejidos renales para análisis bioquímico y los tejidos restantes se tomaron para análisis histopatológico e inmunohistoquímico.
ResultadosEn el grupo LPS, congestión y encogimiento glomerular, degeneración de túbulos distales, infiltración de células mononucleares, restos celulares y acumulación proteica intensa en los túbulos, aumento de las expresiones de Cas-3, factor nuclear kappa beta-p65 (NF-kB p65), los niveles de creatinina, TOS, OSI y niveles disminuidos de TAS, AQP-1 se encontraron significativamente. El tratamiento con IRN invirtió todos estos parámetros. Los niveles restaurados de AQP-1 de IRN por sus efectos antiinflamatorios, antioxidantes y antiapoptóticos debido a la inhibición de la expresión de NF-kB.
Conclusióneste estudio sugiere que la IRN se puede usar en afecciones que afectan los riñones, como la LRA. Se necesitan más estudios para la investigación molecular detallada de IRN en diferentes dosis y duraciones.
The human body has a complex structure and remains a mystery today. This structure works in a physiologically regular system. In case of any disruption, various protection mechanisms come into play. Inflammation plays a role in the basis of this defense mechanism. The inflammation process occurs with tissue damage and continues with the repair process.
Lipopolysaccharide (LPS) is a component of the outer membrane surface found throughout gram-negative bacteria.1 LPS stimulates natural or innate immunity in eukaryotic species strongly.2 LPS binds to macrophages, dendrites, monocytes, and B cells in the blood, leading to the production of transcription factors such as nuclear factor kappa B (NF-κB), and thus the release of inflammatory cytokines.3 The more LPS gets into the blood, the more likely it is to trigger inflammation and cause systemic effects. The kidneys are one of the organs exposed to this condition. When structural damage occurs in the kidney, the inflammation process begins. As a result of inadequate kidney functions in response to inflammation, blood urea nitrogen (BUN) and creatinine (cre) levels begin to arise in plasma. Also, there are water channels in kidney tubules which related with inflammation, especially aquaporin-1 (AQP-1).4 AQP-1 is also involved in the reabsorption of water in the proximal tubule and the thin descending limb of the loop of henle.5 As a result of their damage when inflammation occurs, problems may arise in functions of the kidney such as regulating blood pressure, concentrating urine, and regulating fluid balance.6
One of the pathways involved in kidney inflammation is the NF-κB pathway. The NF-κB complex, which is located in the cytoplasm in an inactive state, becomes active during inflammation and releases inflammatory cytokines, thus initiating the inflammation mechanism. NF-κB-p65 is a subunit of NF-κB transcription complex which has important role in inflammation, cell growth, oxidative stress, immunity, tumorigenesis and apoptosis.7,8
The inadequacy of antioxidant agents that try to compensate for the reactive oxygen molecules that occur due to damage during inflammation cause an insufficiency of antioxidant response to oxidant molecules which can evaluate with total oxidant status (TOS), total antioxidant status (TAS) and oxidative stress index (OSI) in oxidative stress.9,10 In cases where inflammatory damage cannot be tolerated, the apoptosis pathway, which is programmed cell death, can be activated. Caspases (Cas), especially cas-3, play a key role in the occurrence of apoptosis, as they are both initiators and implementers.11 By removing the damaged cells due to apoptosis, further complications can be prevented.
Irbesartan (IRN) is a non-peptide angiotensin receptor antagonist that can be used alone or in combination in hypertension and hypertensive patients with type 2 diabetes-related nephropathy.12 Renin–angiotensin system which results with activation of angiotensin II type 1 (AT1) receptor and increase aldosterone levels to tolerate volume load.13 The main function of IRN is to block the AT1 receptor located in the renin–angiotensin system.14 Thus, it prevents vasoconstriction and reduces the volume load via direct blocking AT1 receptors and decreasing aldosterone levels.15 Studies showed that IRN also has an anti-inflammatory, antioxidant and anti-apoptotic properties.16
Irbesartan and other angiotensin II blockers have been reported to have significant effects in improving kidney function and reducing kidney inflammation. Irbesartan has been shown to have a nephroprotective effect in reducing acute nephrotoxicity in rats via modulation of oxidative stress and antioxidant capacity.17–18 It has been stated that combined treatment with linagliptin and irbesartan protects the kidneys, improves renal functions by inhibiting inflammatory and oxidative stress responses, and shows positive clinical effectiveness in the treatment of diabetic nephropathy.19
The aim of this study is to evaluate the protection of IRN through the NF-kB pathway in LPS induced kidney damage and to question the utility of IRN as a therapeutic agent in possible kidney damage.
Material and methodsAnimalsAdult male Wistar albino rats weighing 250–300g were purchased from the Animal Research Laboratory of Suleyman Demirel University. Animals were allowed to acclimatize for at least seven days before experimentation. Rats were group-housed (eight rats per cage) under a 12:12h light:dark cycle at room temperature (24±1°C) with a relative humidity of 50±10% with access to food and water ad libitum. All experimental procedures were performed following the guidelines for animal research from the National Institutes of Health and were approved by the Committee on Animal Research at Suleyman Demirel University, Isparta (Ethic No: 06.01.2022-01-06).
Experimental proceduresA total of 24 rats were randomly divided into four groups as follows: (1) control group, which was administered 0.5ml saline intraperitoneally (ip). One hour after this application 0.5ml saline was administered again ip. (2) LPS group, which was administered 5mg/kg ip LPS (048K4126, Sigma Aldrich, ABD). One hour after this application 0.5ml saline was administered ip. (3) LPS+IRN group, which was administered 5mg/kg ip LPS (048K4126, Sigma Aldrich, ABD). One hour after this application 3mg/kg IRN was administered ip.
Animals were sacrificed 5h after the last drug administration under ketamine (80–100mg/kg) (Alfamin, Alfasan IBV) and 8–10mg/kg xylazine bio 2% solution (Bioveta, Czech Republic) anesthesia. Kidney tissues were then removed. One part of the tissue was collected and stored at −20°C to measure TAS, TOS, OSI levels. The remaining part of the tissue was fixed in 10% buffered formaldehyde for histopathological examination and to evaluate tumor necrosis factor-alpha (TNF-α), Cas-3, AQP-1, NF-κB expressions by immunohistochemical analysis.20
Biochemical analysisBiochemical analyses included measurements of TAS, TOS, and OSI levels.20 Kidney tissue samples were homogenized with the Ultra Turrax Janke & Kunkel T-25 homogenizer (IKA® Werke, Germany) for oxidant-antioxidant analysis. The TAS and TOS were measured spectrophotometrically (Beckman Coulter AU 5800, Beckman Coulter, USA) using commercial kits (Rel Assay Diagnostics, Gaziantep, Turkey), and OSI values were calculated using the formula OSI=TOS/TAS.21
In the TAS analysis, which determined the sample antioxidative effect against the potent free radical reactions triggered by the produced hydroxyl radical, antioxidants in the sample reduce dark blue-green colored 2,2′-azino-bis (3-ethylbenzthiazoline-6 sulphonic acid; ABTS) radical to a colorless reduced ABTS form. The absorbance change at 660nm was linked with the TAS level of the sample. The results are expressed as millimolar Trolox equivalents per liter (mmolTrolox Equiv./l).22 In the TOS analysis, oxidants found in the sample oxidized the ferrous iondianisidine complex to the ferric ion. The oxidation reactions were raised with glycerol molecules of the reaction medium. The ferric ion forms a colored complex with xylenol orange in an acidic medium. The intensity of the color is related to TOS molecules in the sample and can be measured spectrophotometrically. This assay was calibrated with hydrogen peroxide (H2O2), and results are expressed as micromolar H2O2 equivalents per liter (μmolH2O2Equiv./l).23
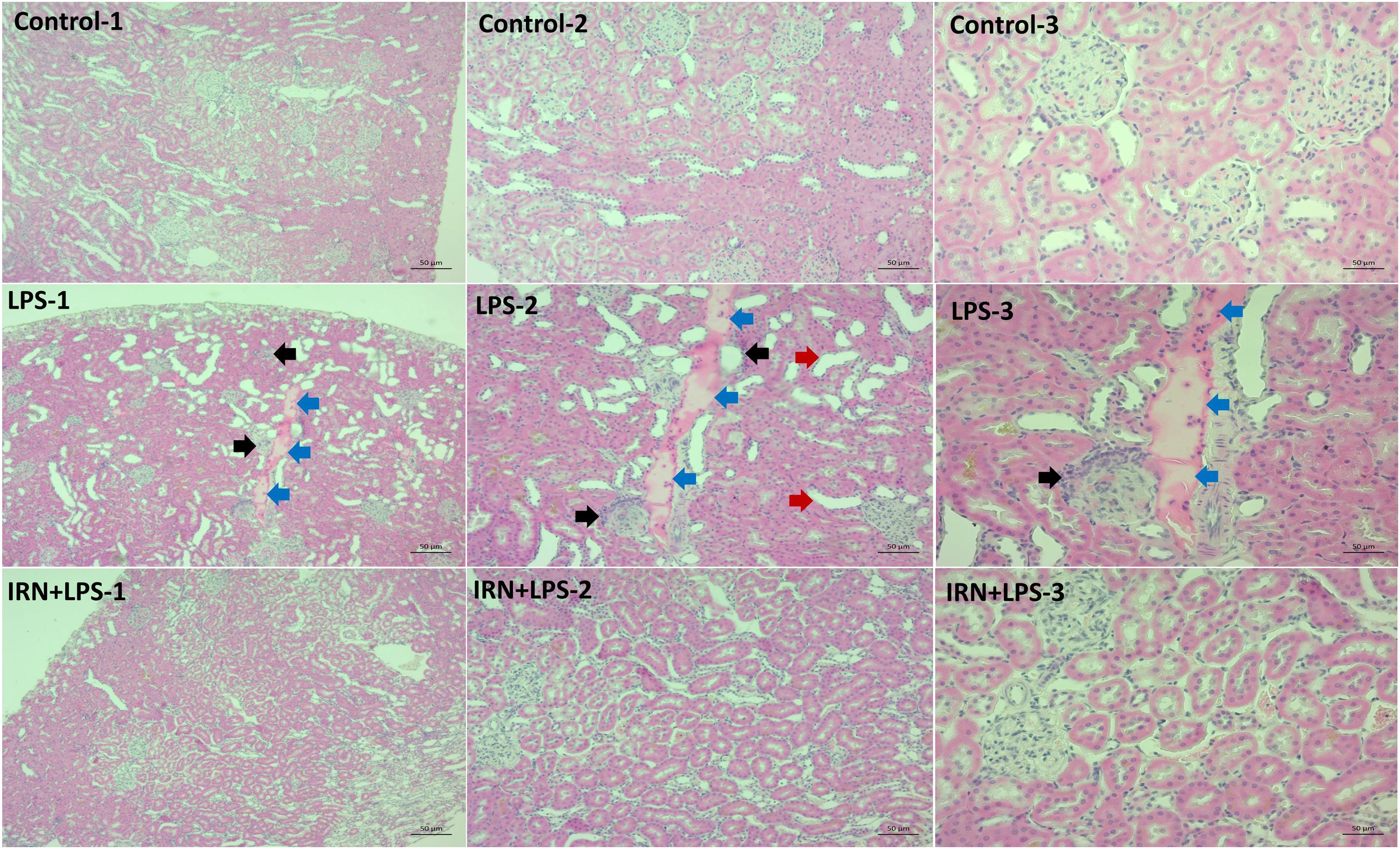
Histopathological analysisDuring the necropsy, kidney samples were fixed in 10% buffered formalin and taken for routine pathology processing procedures by an automatic tissue processor (Leica ASP300S, Wetzlar, Germany) and embedded in paraffin wax. Sections of 5-μm thickness were taken from the paraffin blocks with a rotary microtome (Leica RM2155, Leica Microsystems, Wetzlar, Germany). The sections were then stained with hematoxylin-eosin (HE), mounted with a coverslip, and examined under a light microscope. Histopathological analyses of the kidney were based on quantitative and qualitative changes. Glomerular congestion and shrinkage, degeneration in the distal tubules, mononuclear cell infiltration, areas of cellular debris and dense protein accumulation in the tubules were shown by taking the score table into consideration.
Immunohistochemical analysisTwo series of sections taken from all blocks drawn on poly-l-lysine-coated slides were stained for AQP-1 (Catalog no: bs-1506R, Bioss), Caspase-3 (Catalog no: FNab01289, Fine Test) and NFkB p65 (Catalog no: bs-20159R, Bioss) expression by the streptavidin biotin technique according to the manufacturer's instructions. The sections were incubated with the primary antibodies for 60min, and immunohistochemistry was carried out using biotinylated secondary antibody and streptavidin-alkaline phosphatase conjugate. EXPOSE Mouse and Rabbit Specific HRP/DAB Detection IHC kits (ab80436; Abcam, Cambridge, UK) were used for secondary antibodies. Diaminobenzidine (DAB) was used as the chromogen. For negative controls, an antigen dilution solution was used instead of the primary antibody. All examinations were performed on blinded samples by a specialized pathologist from another university.
For immunohistochemical analyses, sections were separately investigated for each antibody. To evaluate the severity of the immunohistochemical reaction of cells with markers, semiquantitative analysis was performed using a grading score ranging from 0 to 3 as follows: 0=negative, 1=focal weak staining, 2=diffuse weak staining, and 3=diffuse strong staining. For evaluations, 10 different areas in each section were examined under a 40× objective magnification. Morphometric analyses and microphotography were performed using the Database Manual cellSens Life Science Imaging Software System (Olympus Co., Tokyo, Japan). The results were saved and statistically analyzed.
Statistical analysisStatistical analysis was performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Number, percentage, mean, and standard deviation were used to express the measurement values in the experimental and control groups. Since the data were normally distributed, the independent samples T test was used to compare two independent groups, and one way ANOVA analysis was performed to analyze three or more groups. A p<0.05 value was deemed statistically significant. In order to determine which group caused this difference, the “post hoc” Bonferroni test, which is one of the multiple comparison tests, was applied.
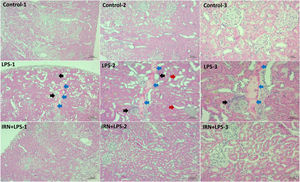
ResultsHistopathology findingsAs a result of the light microscopic evaluation of the kidney tissue preparations of the control group, no histopathological findings were found. Much more histopathological differences were detected in the kidney tissues of the rats in the LPS group compared to those in the control group. In LPS group, glomerular congestion and shrinkage, degeneration of distal tubules, mononuclear cell infiltration, cellular debris and intense proteinous accumulation in the tubules were observed compare with the control group (p<0.001, for all). In LPS+IRN groups, all scores were found to be lower but only glomerular congestion and shrinkage, cellular debris scores, deposition in tubules were statistically significant (p=0.045, p=0.006 and p=0.023 respectively) compare with the LPS group (Fig. 1). When the LPS+IRN group was compared with the control group, it was observed that there was a significant increase in all five scores of histopathological changes (p<0.001, p<0.01, p<0.05, p<0.001 and p<0.001, respectively) (Fig. 1) (Table 1).
Histopathological appearance of the kidneys between the groups. Groups: Control-1, Control-2 and Control-3, LPS-1, LPS-2, LPS-3 and IRN+LPS-1, IRN+LPS-2, IRN+LPS-3. Histopathological appearance of the kidneys between the groups. No histopathological findings were found in the control group. In the LPS-1, LPS-2 and LPS-3 group, glomerular congestion and shrinkage (black arrow), degeneration of the distal tubules (red arrow), mononuclear cell infiltration (black arrow), cellular debris and extensive proteinaceous accumulation in the tubules (blue arrow) were observed. It was observed that it was close to control in IRN+LPS-1, IRN+LPS-2, IRN+LPS-3 (A:X5, B:X10 and C:X20 magnification, scale bar=50μm). The differences between the groups are shown in the score table (Table 1).
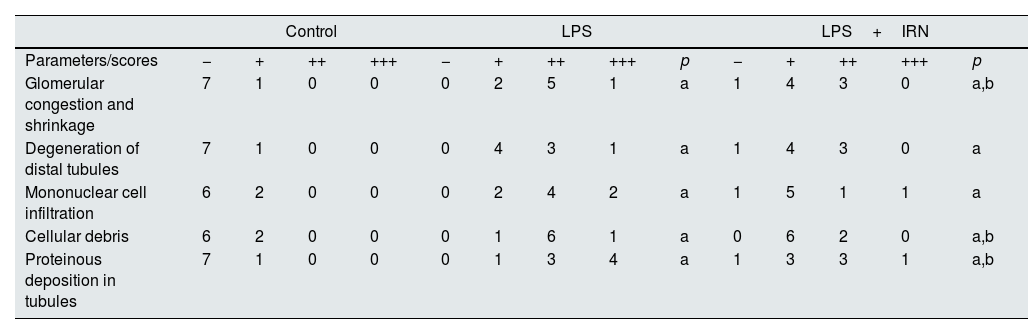
Scores of kidney tissues histopathologic analysis.
| Control | LPS | LPS+IRN | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters/scores | − | + | ++ | +++ | − | + | ++ | +++ | p | − | + | ++ | +++ | p |
| Glomerular congestion and shrinkage | 7 | 1 | 0 | 0 | 0 | 2 | 5 | 1 | a | 1 | 4 | 3 | 0 | a,b |
| Degeneration of distal tubules | 7 | 1 | 0 | 0 | 0 | 4 | 3 | 1 | a | 1 | 4 | 3 | 0 | a |
| Mononuclear cell infiltration | 6 | 2 | 0 | 0 | 0 | 2 | 4 | 2 | a | 1 | 5 | 1 | 1 | a |
| Cellular debris | 6 | 2 | 0 | 0 | 0 | 1 | 6 | 1 | a | 0 | 6 | 2 | 0 | a,b |
| Proteinous deposition in tubules | 7 | 1 | 0 | 0 | 0 | 1 | 3 | 4 | a | 1 | 3 | 3 | 1 | a,b |
Histological changes: (−) score (negative score), no structural damage; (+) score (one positive score), minimal damage; (++) score (two positive scores), middle damage; (+++) score (three positive scores): severe damage.43 The relationships between groups and results of immunohistochemical scores are assessed by the One-way ANOVA test (post hoc LSD test). LPS: lipopolysaccharide, IRN: irbesartan. a: p≤0.05; compared with the control group. b: p≤0.05; compared with the LPS group.
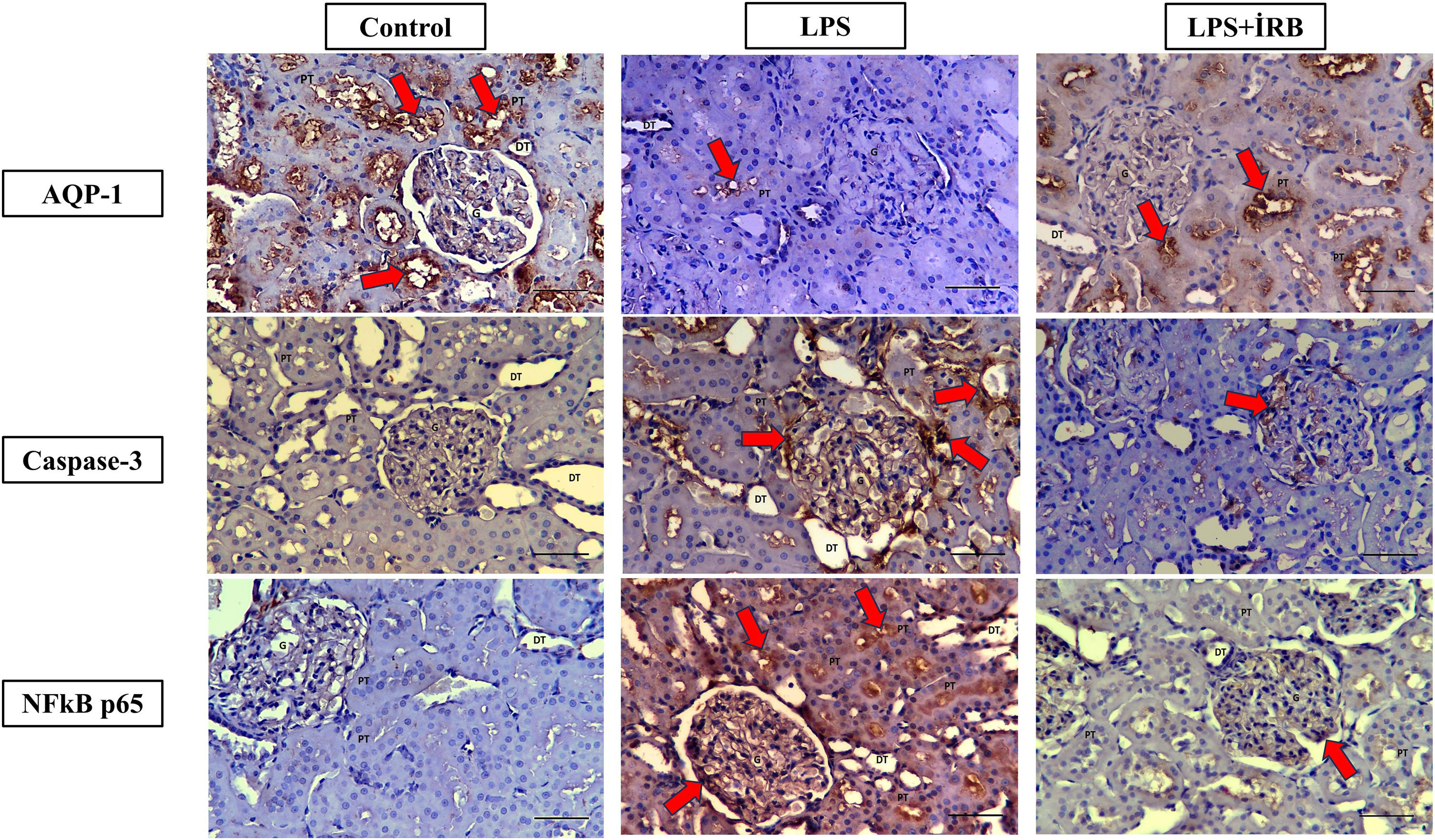
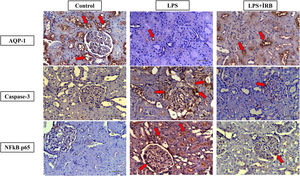
In the control group (+++ grade) AQP-1 immunoreactivity was observed in the cortex region, proximal tubule apical membrane, glomeruli and medulla region. In the LPS group, (+ grade) AQP-1 immunoreactivity was detected in the proximal tubule apical membrane and (++ grade) AQP-1 immunoreactivity was detected in the proximal tubule apical membrane in the LPS+IRN group (Fig. 2) (Table 2).
Immunoreactivities of AQP-1, Cas-3, NF-κB on kidney tissues. Groups: Control (AQP-1, Caspase-3 and NFkB p65), LPS (AQP-1, Caspase-3 and NFkB p65) and LPS+IRN (AQP-1, Caspase-3 and NFkB p65). LPS (AQP-1, Caspase-3 and NFkB p65), LPS (AQP-1, Caspase-3 and NFkB p65) and LPS+IRN (AQP-1, Caspase-3 and NFkB p65). AQP-1, CAS-3 and NF-kB p65 immunoreactivity in the proximal tubule (PT), glomeruli (G) and distal tubule (DT) in the medulla and cortex in control, LPS and LPS+IRN groups. Regions of immunoreactivity are indicated by red arrows. (DABx20, scale bar=100μm). The differences between the groups are shown in the score table (Table 2).
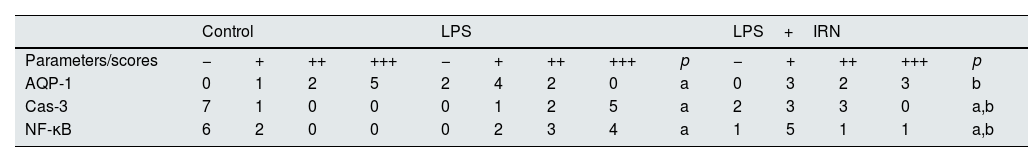
Immunoreactivity scores of AQP-1, Cas-3 and NF-kB of kidney tissues.
| Control | LPS | LPS+IRN | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters/scores | − | + | ++ | +++ | − | + | ++ | +++ | p | − | + | ++ | +++ | p |
| AQP-1 | 0 | 1 | 2 | 5 | 2 | 4 | 2 | 0 | a | 0 | 3 | 2 | 3 | b |
| Cas-3 | 7 | 1 | 0 | 0 | 0 | 1 | 2 | 5 | a | 2 | 3 | 3 | 0 | a,b |
| NF-κB | 6 | 2 | 0 | 0 | 0 | 2 | 3 | 4 | a | 1 | 5 | 1 | 1 | a,b |
Histological changes: (−) score (negative score), no structural damage;(+) score (one positive score), minimal damage; (++) score (two positive scores), middle damage; (+++) score (three positive scores): severe damage.43 The relationships between groups and the results of immunohistochemical scores are assessed by the One-way ANOVA test (post hoc LSD test). LPS: lipopolysaccharide, IRN: irbesartan, AQP-1: aquaporin-1, Cas-3: caspase-3, NF-kB: nuclear factor kappa beta. a: p≤0.05; compared with the control group. b: p≤0.05; compared with the LPS group.
While Cas-3 immunoreactivity was not observed in the control group, (+++ grade) Cas-3 immunoreactivity was observed in the cortex region, proximal tubule apical membrane, glomeruli and distal tubule in the LPS group. In the LPS+IRN group, proximal tubule apical membrane, glomeruli and distal tubules Cas-3 immunoreactivity was detected (+ grade) (Figure 2) (Table 2).
NF-κB p65 immunoreactivity was not observed in the control group. (+++ grade) NF-κB p65 immunoreactivity was observed in the proximal tubule apical membrane, glomeruli and distal tubule in the LPS group. In addition, lymphatic clusters were observed in the medulla region in the LPS group and (+++ grade) NF-κB p65 immunoreactivity were noticeable different in this region. In the LPS+IRN group, (+ grade). NF-κB p65 immunoreactivity was detected in the proximal tubule apical membrane and glomeruli (Fig. 2) (Table 2).
AQP-1 immunoreactivity scores decreased in the LPS group compared to the control group and IRN reversed AQP-1 levels significantly (p<0.001 and p<0.05, respectively). There was no statistically significance observed between control and LPS+IRN groups. In the LPS group Cas-3 immunoreactivity scores were found to be higher compared to control and IRN attenuated Cas-3 levels significantly (p<0.001 for both). Significant increment of Cas-3 level detected in the LPS+IRN group compared control group (p<0.01). In LPS and LPS+IRN groups NF-κB scores were increased significantly compared to control group (p<0.001 and p<0.01 respectively). NF-κB scores were significantly decreased in LPS+IRN group in comparison to LPS group (p<0.01) (Fig. 2) (Table 2).
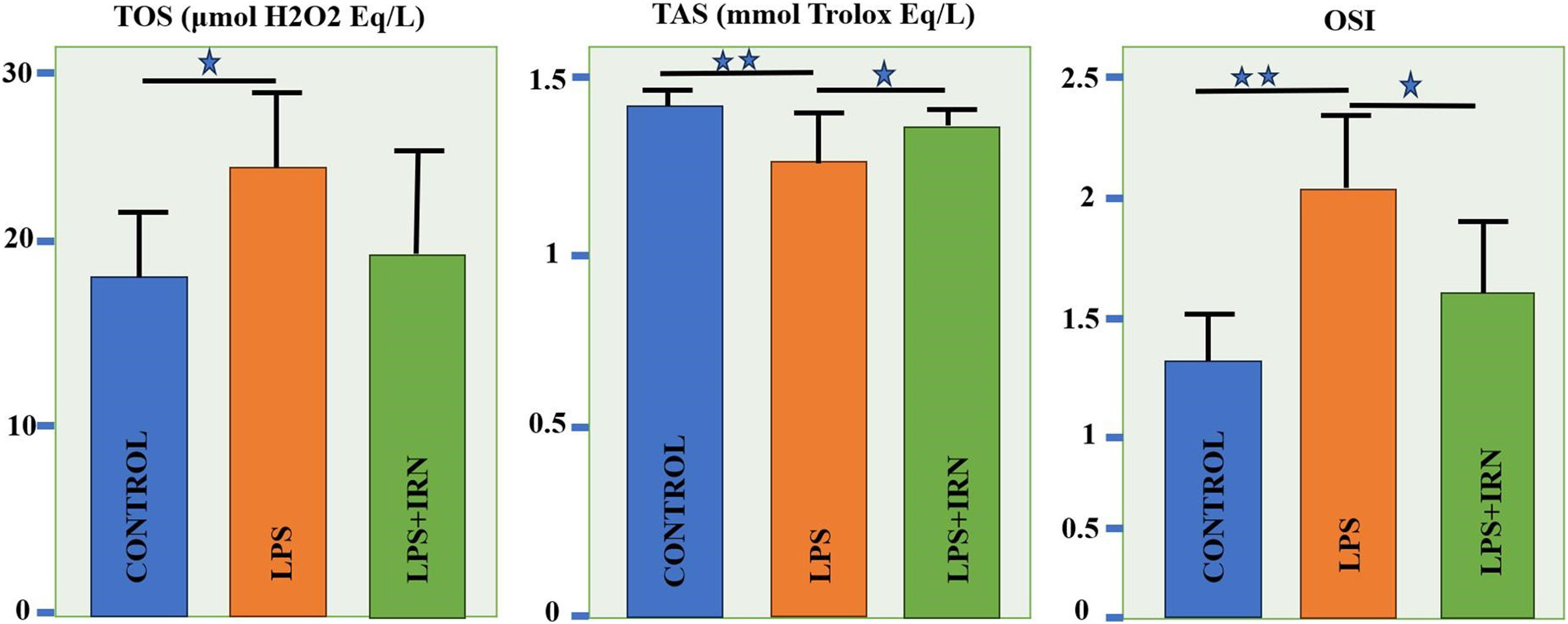
Biochemical resultsTOS levels were increased significantly in the LPS group compared to the control group (p<0.05). IRN treatment decreased TOS levels, but these results were not significant. The mean TAS levels of the LPS group were found to be lower compared to control group and IRN treatment reversed it significantly (p<0.01 and p<0.05, respectively). In the LPS group, mean OSI levels increased significantly compared to control group (p<0.01). When the LPS+IRN group was compared with the LPS group, it was observed that there was a significant decrease in OSI levels (p<0.05) (Fig. 3).
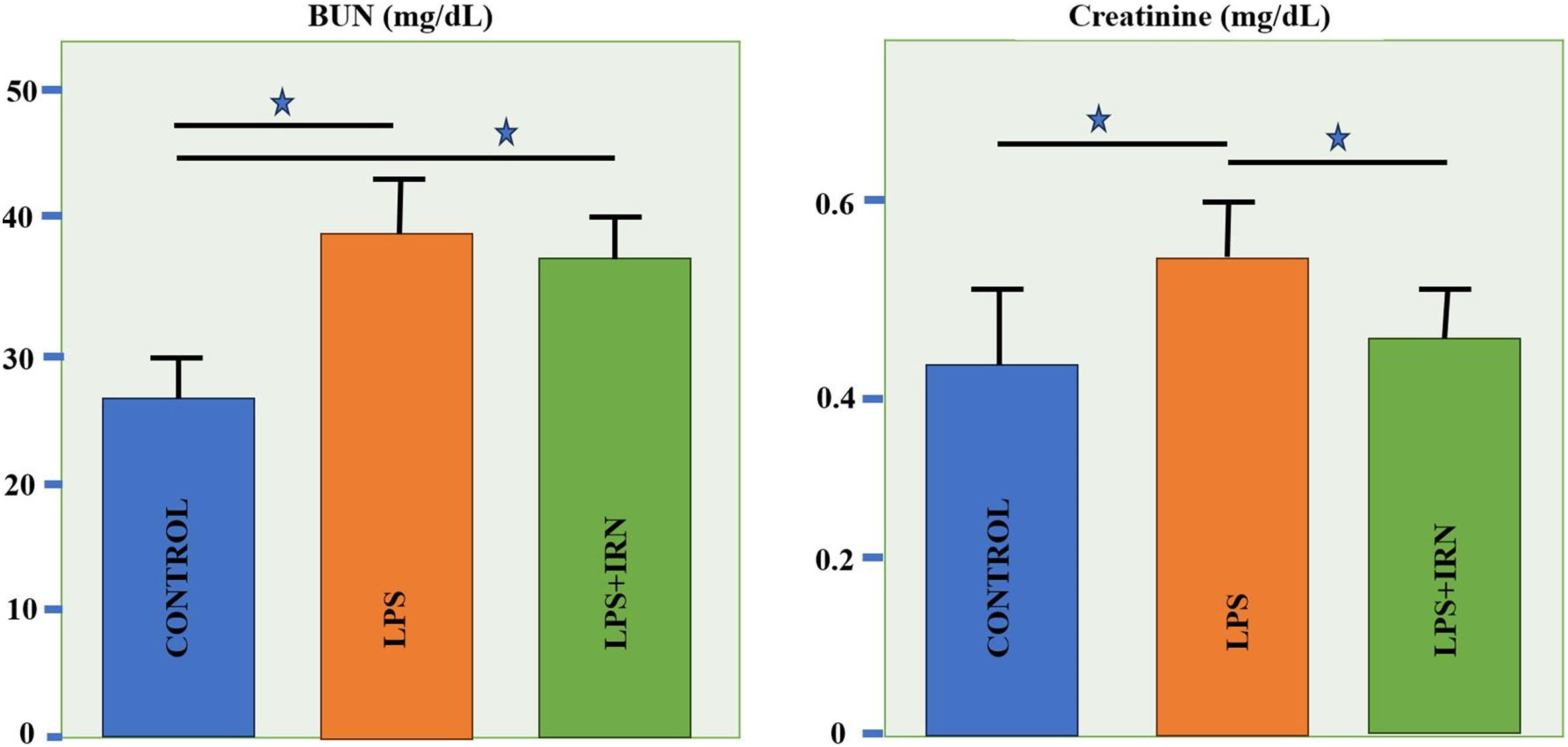
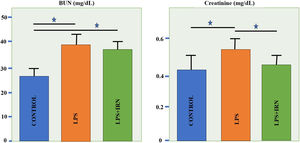
Mean Cre levels have shown to be increased in LPS group compared to control group (p<0.05). In LPS+IRN group, IRN reversed this parameter (p<0.05) (Fig. 4). Mean BUN levels increased significantly in LPS and LPS+IRN group compared to control group (p<0.05 for both). In LPS+IRN group treatment lowered BUN levels in comparison to LPS group, but it was not significant.
DiscussionHypertension is a systemic disease that can cause problems in many organs, especially the kidney, due to vascular damage.24 Agents that block the renin–angiotensin system are frequently used in the antihypertensive treatment approach to reduce blood pressure, vasodilation, suppression of the sympathetic system, and reduction of intravascular volume.25 Among them, drugs such as IRN, which block the angiotensin II receptor AT-1, also have tissue protective effects due to their anti-inflammatory, antioxidant and anti-apoptotic properties.26
A study has shown that bone marrow-derived macrophage activation of the angiotensin II type 2 receptor (AT2R) contributes to anti-inflammatory and partial renoprotection against the early stages of LPS-induced AKI. Moreover, the effect of AT2R on anti-inflammatory mechanisms associated with renal protection has been noted.27
Inflammation and oxidative stress play an important role in kidney tissue damage.28 Activation of these two mechanisms by their specific receptors on the cell surface may trigger some intracellular pathways with signal transduction.29 In particular, the expression and activation of proteins such as NF-κB, which have a central role in intracellular mechanisms, stimulate the cell nucleus, causing the synthesis of markers such as TNF-α and Cas-3, and triggering the inflammatory response throughout the tissue, causing the damage to expand.20
Considering the results of this study, increased NF-κB levels and histopathological responses in the kidney tissues of the damage group show that an inflammatory scene develops at the tissue level. It can be said that the findings of glomerular congestion and shrinkage, degeneration of distal tubules, mononuclear cell infiltration, cellular debris and intense proteinous accumulation in the tubules, which were found to be significantly increased in the damage group examined by HE staining compared to the control group, developed due to inflammation. Along with the degenerative changes in these glomerular and tubular structures, the possible decrease in filtration capacity due to intense protein accumulation will cause difficulties in the treatment of diseases such as hypertension that require diuresis. Decreased protein accumulation seen in nephropathies accompanying hypertension with IRN treatment will make an important contribution to the treatment of diseases such as diabetic nephropathy with proteinuria.30 It has been shown that irbesartan reduces acute nephrotoxicity and has a renal protective effect in rats by modulating oxidative stress and antioxidant capacity.18 In this respect, the results of our study are compatible with similar studies.
Aquaporins (AQPs) are transmembrane water channel proteins that regulate urine concentration, osmolarity, and fluid volume.31 There are studies showing that drugs negatively affect aquaporin expression and distribution.32
AQP-1 channels, which are associated with renal diuresis function and carry out water transport, are in protein structure and their expression increases or decreases in various situations.33 For example, Kandemir et al. showed in their study, a decrease was observed in the number of AQP channels with inflammation in the kidneys, while it was found to increase with anti-inflammatory treatment.34 In this study, the restored AQP-1 levels with IRN treatment, which decreased with the development of inflammation, may have enabled the diuretic mechanism to maintain its functionality.
The increase in TNF-α levels, which is one of the most important markers of acute inflammation,35 by immunostaining method and the mononuclear cell infiltration that occurs in the damage area with histological evaluation also provides information about the severity of the scene. From the aspect of evaluating the kidney functions, increased BUN and cre levels guides us about the progression of renal inflammation.36 The ineffectiveness of existing drugs in managing this pathological process has led to its inability to fight emergent and fatal diseases. Therefore, it is very important to reduce the expression of proteins that have a central role in inflammation, such as NF-κB, or to prevent their activation. In this study, effective reduction of NF-κB expressions, which increase together with TNF-α, by IRN shows how effective it can be in preventing acute inflammation.
In a study, histopathological examination of kidney tissue in the LPS group showed glomerular capillary dilation, renal interstitial edema, deformation and shedding of renal tubular epithelial cells, renal tubular damage and apoptotic cells. In addition, it has been reported that serum BUN, TNF-α, IL-1β and NF-κB levels increased.37 In this respect, the results of our study are consistent with the conducted study.
It is known that inflammation and oxidative stress trigger each other.38 As it is known, oxidative stress occurs as a result of insufficient antioxidant response to the damage caused by oxidant substances in the tissue.9 In order to demonstrate this, the levels of oxidative stress parameters TOS, TAS and OSI are examined, as they reflect a holistic perspective in the studies39 In this study, a statistically significant increase was found in the LPS group in TOS and OSI values, which are indicators of oxidative stress. However, the insignificant decrease in TOS values in the treatment group may be an indication that IRN treatment needs longer-term treatment to reduce oxidant levels in oxidative stress. It is known that apoptosis, which is programmed cell death, is also triggered by oxidative stress and inflammation.40
In a study evaluating endotoxin-induced AKI, it was shown that Cas-3 activation increased in the experimental group and apoptotic cells were observed.41 In another study in which sepsis-induced acute kidney injury was caused by LPS, it was shown that it increased the number of apoptotic cells and activated the expression of proteins (cleaved Caspase-3 and Bax) associated with pro-apoptotic cells.42 The parallelism of this mechanism with the immunohistochemically demonstrated Cas-3 levels in the damage group proves the above-mentioned situation. Reducing the increased Cas-3 levels by the IRN shows that the drug also has an anti-apoptotic effect.
ConclusionsInflammation, oxidative stress and apoptosis occurring in acute kidney injury secondary to various systemic diseases cause many pathological events at the cellular level. The antioxidant, anti-inflammatory and anti-apoptotic properties of IRN, which is known to have an antihypertensive effect, have been proven in this study. However, further studies are needed to determine the effects of IRN on clinical acute kidney injury with detailed molecular studies and longer periods of use.
Ethical statementsIn this study, experimental animal (rat) was used. The necessary permissions were obtained from the Animal Experiments Local Ethics Committee of Suleyman Demirel University (SDU) Faculty of Medicine (Ethic No: 06.01.2022-01-06).
FundingNo financial support was received.
Conflicts of interestThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.