Progressive haemodialysis (HD) is a starting regime for renal replacement therapy (RRT) adapted to each patient's necessities. It is mainly conditioned by the residual renal function (RRF). The frequency of sessions with which patients start HD (one or two sessions per week), is lower than that for conventional HD (three times per week). Such frequency is increased (from one to two sessions, and from two to three sessions) as the RRF declines.
Methodology/DesignIHDIP is a multicentre randomized experimental open trial. It is randomized in a 1:1 ratio and controlled through usual clinical practice, with a low intervention level and non-commercial. It includes 152 patients older than 18 years with chronic renal disease stage 5 and start HD as RRT, with an RRF of ≥4ml/min/1.73m2, measured by renal clearance of urea (KrU). The intervention group includes 76 patients who will start with one session of HD per week (progressive HD). The control group includes 76 patients who will start with three sessions per week (conventional HD). The primary purpose is assessing the survival rate, while the secondary purposes are the morbidity rate (hospital admissions), the clinical parameters, the quality of life and the efficiency.
DiscussionThis study will enable us to know, with the highest level of scientific evidence, the number of sessions a patient should receive when starting the HD treatment, depending on his/her RRF.
Trial registrationRegistered at the U.S. National Institutes of Health, ClinicalTrials.gov under the number NCT03239808.
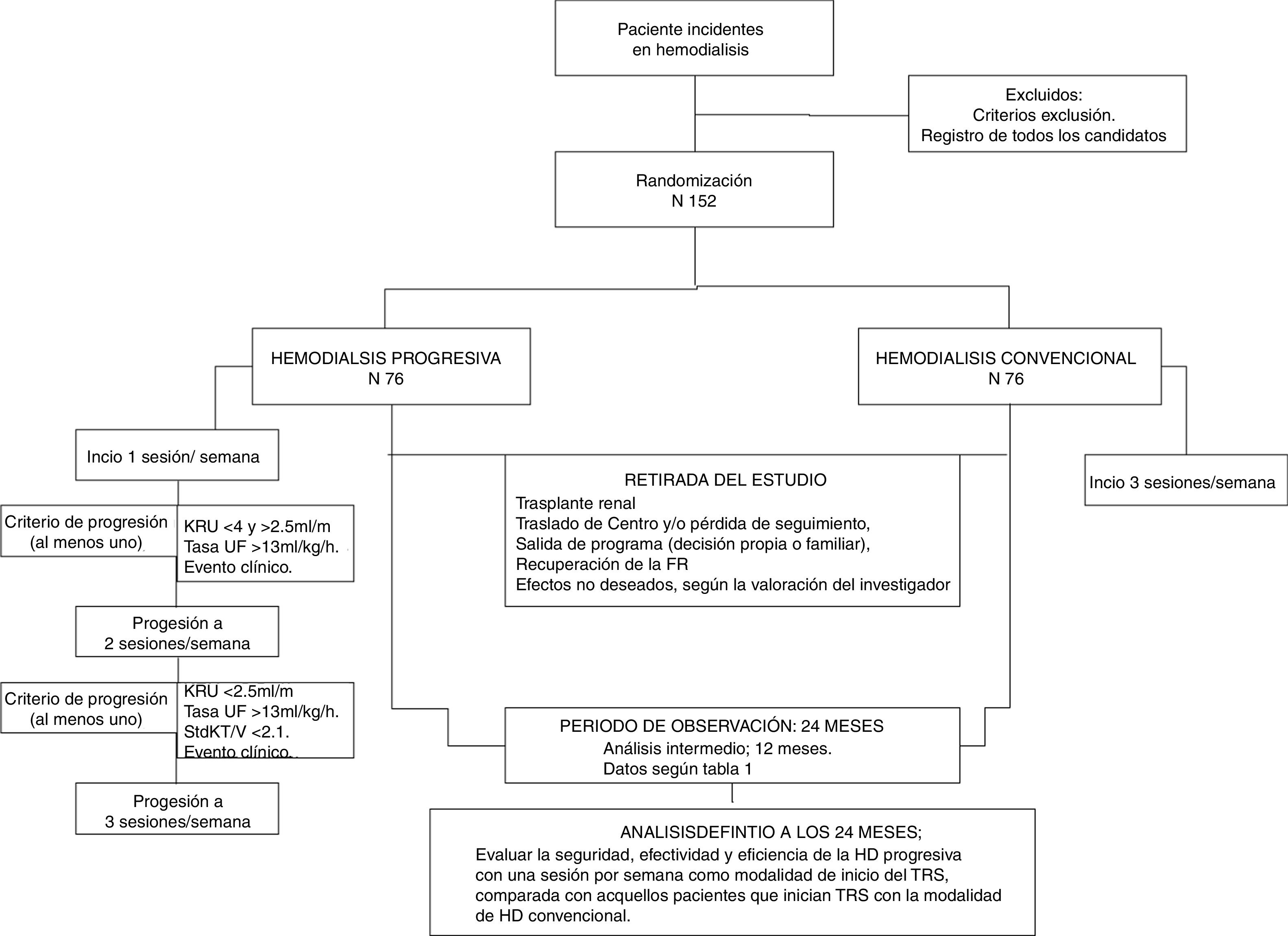
La hemodiálisis (HD) progresiva es una modalidad de inicio del tratamiento renal sustitutivo adaptada a las necesidades individuales de cada paciente. Está condicionada fundamentalmente por la función renal residual (FRR). En ella, la frecuencia de sesiones con las que el paciente inicia HD (una o 2 sesiones por semana) es menor que en la HD convencional (3 por semana). Dicha frecuencia aumenta (de una a 2, y de 2 a 3) con el declinar de la FRR.
Metodología/diseñoDiPPI es un estudio abierto, multicéntrico, experimental, aleatorizado 1:1 y controlado con procedimiento de práctica clínica habitual, de bajo nivel de intervención y no comercial. Incluye 152 pacientes mayores de 18 años, con enfermedad renal crónica estadio 5, que inician HD como tratamiento renal sustitutivo; y la FRR, medida por aclaramiento renal de urea (KrU) es ≥4ml/min/1,73m2. El estudio se basa en un grupo de intervención con 76 pacientes que iniciarán HD con una sola sesión por semana (modalidad progresiva) y un grupo control con 76 pacientes que comenzarán con 3 sesiones por semana. El objetivo primario es evaluar la supervivencia y los objetivos secundarios son la morbilidad (hospitalizaciones), los parámetros clínicos habituales, la calidad de vida y la eficiencia.
DiscusiónEste estudio permitirá conocer, con la máxima evidencia científica, cuántas sesiones debe recibir un paciente al inicio del tratamiento con HD, dependiendo de su FRR.
RegistroRegistrado en U.S. National Institutes of Health, ClinicalTrials.gov con número NCT03239808.
Conventional hemodialysis (HD) of 3 days per week for 3–5h, as outpatient in a health center, is the most widely used modality of renal replacement therapy (RRT)1; however, it has an unacceptably high mortality (10–20% per year).2 In incident patients, the transition to HD during the first year entails even worse results, with the intervention of factors outside the RRT.3 To improve these results, new techniques and modalities based on more dose of HD and/or more sessions2 have been proposed. Nonetheless, recently published randomized controlled trials have shown controversial results in relation to the clinical benefit,4,5 together with a higher rate of vascular access complications,6 and a lower preservation of residual kidney function (RKF).7
The 2015 guidelines of the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (KDOQI)1 allow to reduce the weekly dose of dialysis in patients with urea renal clearance (KrU) greater than 3ml/min/1.73m2. In these cases, the dialysis clearance (Kd) obtained with 2 sessions/week is added to renal clearance (Kr), achieving an adequate dose of dialysis.8,9 It is surprising that few centers follow this recommendation when more than 50% of patients initiate HD with a KrU>3ml/min.10
Authors such as Kalantar-Zadeh et al.10,11 in USA or Teruel et al.,12 in Spain, have published their experience with 2 sessions of HD per week in incident patients. With this modality they manage to preserve RKF and survival rates similar to those obtained with conventional HD. This could be explained by the fact that Kr had a clinical weight greater than Kd,8 since RKF, in addition to maintaining the homeostasis of the internal environment, contributes to the production of vitamin D and erythropoietin,13,14 and to the purification of protein bound uremic toxins which are poorly dialyzed.14,15 We can state that the correlation between RKF and survival is strong and consistent, as well as playing a crucial role in achieving adequate dialysis.1,9,16
Currently, the number of sessions that a patient must have at the initiation of HD is a matter of debate.8,17–19 Progressive HD is an option for initiation of HD which is adapted to the RKF, where the frequency of HD sessions increases as the volume of daily diuresis declines.8,17–19
The study “Evaluation of the Safety and Effectiveness of Progressive Hemodialysis in Incident Patients” (DiPPI)20 aims to determine whether initiating HD with one session per week reduces mortality in incident patients and has an effect on morbidity (hospitalizations), clinical parameters, quality of life and efficiency compared with those patients who initiate RRT with conventional HD.
Method and study designDesignProspective, multicenter, open, randomized and controlled clinical trial with the usual clinical practice, based on starting HD with 3 sessions per week (control group).
InterventionIt consists in reducing the frequency or number of weekly sessions at the initiation of HD. The experimental group will start with a session/week to progress to 2 and then to 3 sessions/week according to progression criteria.
DiPPI does not use drugs or placebos, and the complementary diagnostic or follow-up procedures do not entail risk for the safety of the subjects, being similar to those of the usual clinical practice. Therefore, it is considered a “low level intervention clinical trial”. It is also a “non-commercial clinical research”, as it has been designed directly by the promoters and principal investigators, without any input from the industry.
ParticipantsHospital units and outpatient HD centers from different geographical areas. It will include only incident patients. Patients admitted for intercurrent problems will remain in the assigned group of the study and will be evaluated according to their randomization.
Inclusion criteria- •
Over 18 years of age with chronic kidney disease (CKD) stage 5 who have chosen HD as a the modality of treatment.
- •
RKF measured by KrU21≥4ml/min/1.73m2. In general it is recommended not to start HD with a KrU>7.
- •
Informed consent signed.
- •
Urgent or non-scheduled initiation of HD. It is assumed that this situation does not allow the collection of urine for 24h before the first session, or that had not collected 24h urine in the previous 30 days.
- •
Patients prevalent in other modalities of RRT.
- •
Associated diseases: active neoplasia, cardiorenal or hepatorenal syndrome, active inflammatory disease, cardiovascular disease defined as HYHA class IV heart failure, unstable angina, or ischemic heart disease with admission in the previous 3 months.
Patients in the experimental group will increase from 1–2 weekly sessions, if they meet any of the following criteria:
- •
Decrease in KrU21 (less than 4 and more than 2.5ml/min/1.73m2). This decrease must be confirmed in the following analytical tests obtained the following month.
- •
Inter-session weight gain (weekly) that entails ultrafiltration rates higher than 13ml/kg/h, maintained for 3 weeks.
- •
Clinical event that requires unscheduled sessions (more than one).
Patients with 2 sessions per week will go to conventional HD if:
- •
KrU21 is less than 2.5ml/min/1.73m2, and/or standard Kt/V less than 2.1 weekly. This decrease must be confirmed in the following analytical test obtained the following month.
- •
Inter-session weight gain that conditions an ultrafiltration rate higher than 13ml/kg/h, maintained during 3 sessions.
- •
Clinical event that requires unscheduled sessions for resolution.
- •
Recruitment period: 18 months since the inclusion of the first patient. Patients selected as candidates will be registered in the patients form (Appendix A). If they meet the eligibility criteria and sign the informed consent, they will be randomized.
- •
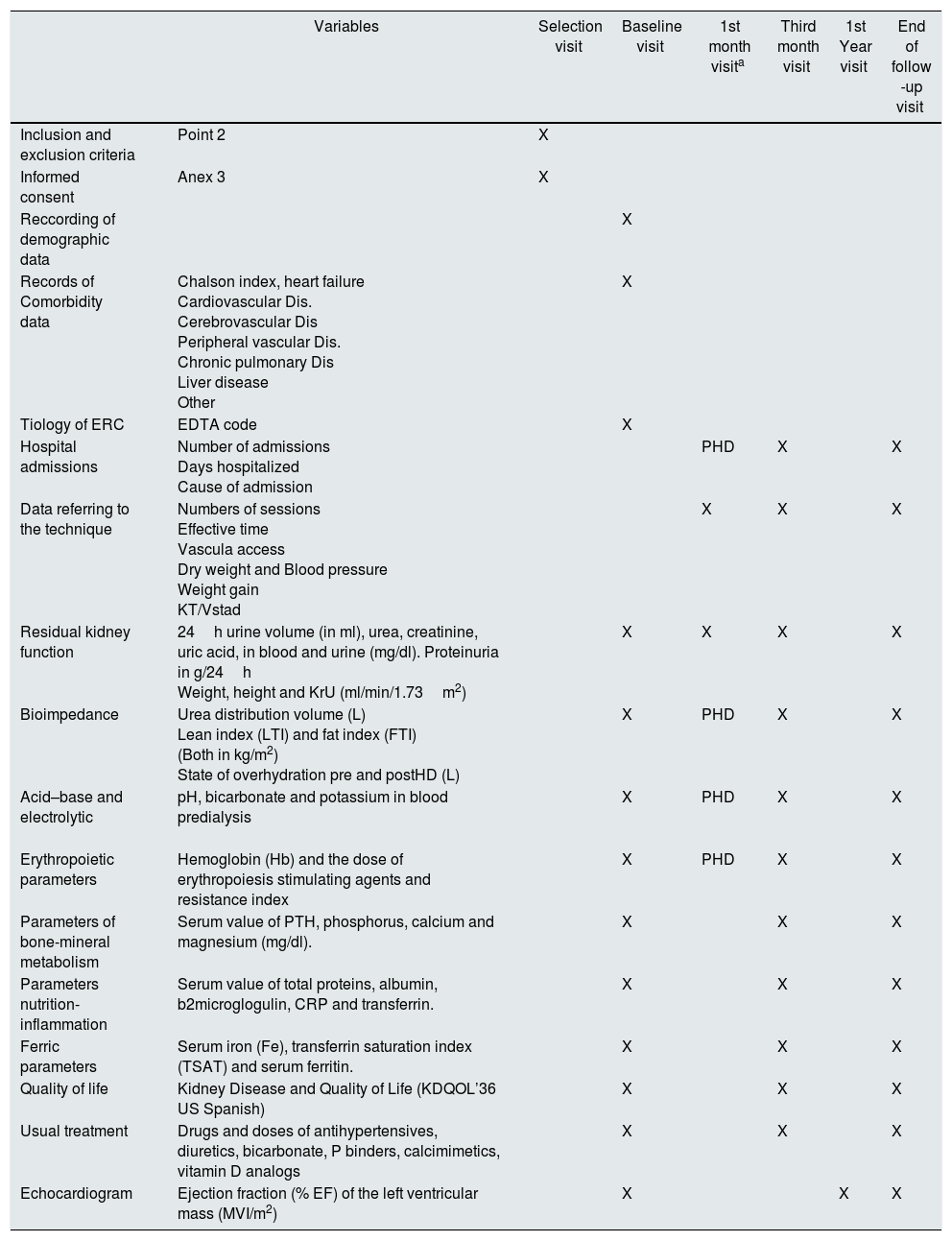
Follow-up period: 24 months. In it biochemical determinations and diagnostic tests will be performed with the periodicity that appears in the calendar of visits (Table 1). Patients in the experimental group, when they progress to 3 sessions/week, will perform the same visits as the control group. The work scheme is defined in Table 1.
Table 1.Organization chart of study visits.
Variables Selection visit Baseline visit 1st month visita Third month visit 1st Year visit End of follow -up visit Inclusion and exclusion criteria Point 2 X Informed consent Anex 3 X Reccording of demographic data X Records of Comorbidity data Chalson index, heart failure
Cardiovascular Dis.
Cerebrovascular Dis
Peripheral vascular Dis.
Chronic pulmonary Dis
Liver disease
OtherX Tiology of ERC EDTA code X Hospital admissions Number of admissions
Days hospitalized
Cause of admissionPHD X X Data referring to the technique Numbers of sessions
Effective time
Vascula access
Dry weight and Blood pressure
Weight gain
KT/VstadX X X Residual kidney function 24h urine volume (in ml), urea, creatinine, uric acid, in blood and urine (mg/dl). Proteinuria in g/24h
Weight, height and KrU (ml/min/1.73m2)X X X X Bioimpedance Urea distribution volume (L)
Lean index (LTI) and fat index (FTI)
(Both in kg/m2)
State of overhydration pre and postHD (L)X PHD X X Acid–base and electrolytic
pH, bicarbonate and potassium in blood predialysis X PHD X X Erythropoietic parameters Hemoglobin (Hb) and the dose of erythropoiesis stimulating agents and resistance index X PHD X X Parameters of bone-mineral metabolism Serum value of PTH, phosphorus, calcium and magnesium (mg/dl). X X X Parameters nutrition-inflammation Serum value of total proteins, albumin, b2microglogulin, CRP and transferrin. X X X Ferric parameters Serum iron (Fe), transferrin saturation index (TSAT) and serum ferritin. X X X Quality of life Kidney Disease and Quality of Life (KDQOL’36 US Spanish) X X X Usual treatment Drugs and doses of antihypertensives, diuretics, bicarbonate, P binders, calcimimetics, vitamin D analogs X X X Echocardiogram Ejection fraction (% EF) of the left ventricular mass (MVI/m2) X X X In the data referring to the technique, if there are several parameters (for example TA, weight gain, etc.), the value recorded will correspond to the session in which the analytical measurements are obtained.
Withdrawal from the study: any patient will be withdrawn from the study due to the following: recovery of the RF, kidney transplant, loss of follow-up, exit from the program and withdrawal of consent. In these cases, the follow-up visit will be carried out and no replacement will be made.
ObjectivePrimary objective- •
Survival. Study period: 2 years.
- •
Hospitalizations for any reason. Study period: 2 years.
- •
Preservation of the RKF. Study period: 2 years.
- ∘
Reduction of glomerular filtration rate (GFR) and tubular function.
- ∘
Average volume of diuresis and percentage of patients in anuria (volume≤200ml/day) in 2 consecutive measurements.
- •
Adequacy parameters. Study time: 3, 6, 12 months and 2 years.
- -
-Control of anemia. Patients with hemoglobin levels in the therapeutic range (expressed in %) and average levels of the erythropoietin resistance index (ERI in IU/kg/week).
- -
Control of bone-mineral metabolism. Mean levels of calcium, phosphorus and intact parathyroid hormone and percentage of patients within therapeutic range.
- -
-Control of specific heart disease. Study time: 12 and 24 months. Left ventricular ejection fraction (LVEF). Percentage of patients with a left ventricular mass index (LVMI) adjusted to body surface ≥125g/m2, or with pericardial effusion.
- -
Control of Quality of life. Score obtained in the validated Kidney Disease and Quality of Life survey (KDQOL’36 SF).
- -
-Cost effectiveness of the intervention: expressed as an increase in cost per additional year gained, adjusted to the quality of life.
It was calculated to detect differences in the contrast of the null hypothesis Ho: the ratio between the medians of the survival time is not inferior to the noninferiority limit, by means of a log-rank test for 2 independent samples (of non-inferiority in a function of exponential survival). For this, it is necessary to include 152 patients in the study, with a 1:1 randomization, that is, 76 patients in each group, assuming the following parameters:
- -
Inclusion period of 18 months.
- -
Maximum duration of the follow-up period, 24 months.
- -
Median survival in the conventional HD group, 74 months.
- -
Average of the time until the censoring, 12 months.
- -
Non-inferiority limit of 4 months.
- -
Error type I 5% (significance).
- -
Error type II 20% (power).
There is a centralized single list. It has 152 randomization codes (sample size) and an additional 24 in case that more patients are included. It has 2 stratus: according to age (>o<75 years), and according to the basal KrU (≥o<5.5ml/min/1.73m2). This randomization is balanced every 6 participants.
The investigator responsible for each center will formally request the randomization to the clinical research office (C.R.O. Delos Clinical) through annex 1.
Centralized prescription of the dialysis doseEach patient will receive a “centralized prescription” of the dose. It will be based on the necessary eKt/V according to the KrU of each patient, to obtain an EKrU of 12-KrUml/min/1.73m2 in a weekly HD and a stdKt/V of 2.3 volumes weekly for 2 times, as published by Casino and Basile.22 All calculations related to the kinetic model of urea (UKM) are based on the prescription tool23 and the Solute–Solver24 software. The control group will receive a dose of spKt/V of 1.4 per session, neglecting the RKF.1
Note: KDOQI1 suggest a stdKt/V=2 weekly volumes for HD programs other than 3 times per week HD. But it is not mention the schedule once a week. Therefore, we adopted the variable goal recently suggested for EKRU as a guide for the week program, which seems to be in accordance with our empirical experience.
VariablesThe data will be obtained from the patient's medical history. Researchers will fill in the electronic data collection notebook (CRDe) in the foreseen periods.
Demographic, clinical data and tests performed. The biochemical determinations, the diagnostic tests and their periodicity are shown in Table 1, and are usually recommended in the guidelines for these patients.
Survival. The follow-up will be determined in days. It will be the difference from the date of the end of follow-up and the date of the baseline visit. Events will be counted as deaths (follow-up less than 24 months) or as an end to follow-up (24 months).
Hospitalizations: number and days of Hospitalizations will be recorded in each patient. Reasons for admission are: infections, problems with the vascular access (performance, repair, replacement, thrombosis or bleeding), heart disease, gastrointestinal bleeding or others.
Preservation of the RKF. The GFR (in ml/min) will be calculated by the half-sum of the clearance of urea and creatinine, and the tubular function by means of the fractional excretion of phosphorus, uric acid and potassium.
Control of anemia. Hemoglobin level (in g/dl) will be measured and doses of erythropoiesis stimulating agents (in IU) will be obtained.
Control of bone-mineral metabolism. Calcium, phosphorus (both in mg/dl) and intact PTH (in pg/dl) will be measured.
Control of specific heart disease. The LVEF (in%), the LVMI (in g/m2) and the presence of pericardial effusion will be assessed.
Quality of life. The items of the KDQOL’36 SF survey will be recorded.
Cost effectiveness of the intervention. The costs during the follow-up will be calculated in each patient. The costs will be counted as: the sessions carried out, at the rate of € 201 per session, the transport to the center at € 20 per session, and the hospital admissions at € 498 per day of admission.25 These rates will not reflect the costs, nor the prices paid for each service. Nor will they be representative of all participating hospitals. However, when used as ratios, it will be possible to calculate which type of HD start is less expensive, and therefore more efficient.
Period of time (Tp) in progressive HD. Each patient in a progressive HD modality will be registered. Will measure the period time from the beginning of the study until progression to 2 sessions per week (Tp in one HD session/week in days). Likewise the period of time in days from the initiation of 2 HD sessions per week until conventional HD or end of the study will be measured (Tp in 2 HD sessions/week in days). The period of time (in days) since the beginning of the study to the initiation of conventional HD or end of the study will also be recorded (Tp in progressive HD in days).
Statistical methodsPopulation to analyzeAll patients included in the study, regardless of their follow-up period, that is, the study population is by intention to treat.
Intermediate analysiAll the objectives will be analyzed in all patients when they reach 12 months of follow-up. In this analysis, methodology and variables will be the same as the analysis to be performed at the end of the follow-up period (Fig. 1).
Descriptive analysisAll variables collected at baseline visit will be evaluated. The qualitative variables will be expressed as percentages and differences will be evaluated using the Chi-square test or the Pearson statistical test if the distribution of observed frequencies is not fulfilled. The quantitative variables will be expressed as mean, median, standard deviation and interquartile range; to assess differences will use the Student's “t” or the Mann–Whitney test if the normal distribution is not met. They will have a level of significance of 5% and a power of 80% for the achievement of the objectives.
Primary objectiveAnalysis of survival. It will be measured by a bivariate analysis or Kaplan–Meier test. The difference between the mean and median survival time, between both branches of the study, will be analyzed by log-rank test. A multivariate analysis or multivariate Cox regression will be performed to assess the real contribution of the intervention (progressive HD) and/or any variable that affects survival.
Secondary objectivesAnalysis of hospital admissions. In each group, the average number of admission and days admitted to the hospital will be calculated. The difference between the means will be evaluated by Student's “t” or its non-parametric Mann–Whitney alternative.
Analysis of the RKF. The changes in GFR, tubular function and the 24h urine volume from baseline during the follow-up will be compared using the Wilconxon test. The period of time maintaining the RKF (volume≥200ml/day) will be evaluated using the Kaplan–Meier technique. To assess the differences between the mean and the median, the log-rank test will be performed. The proportion of patients (in%) with a volume≤200ml/day at the end of the follow-up will be compared by Chi-square test or Pearson's statistical test (according to the distribution of observed proportions).
Other analytical parameters. To compare the percentage of patients with hemoglobin<10.5g/L, or calcium, phosphorus and PTH within the therapeutic range (in each branch of the study), the Chi-square test or the Pearson test will be performed, the later will be used if the frequency distribution is not fulfilled. The differences between mean levels of the resistance index to erythropoietin, calcium, phosphorus and intact PTH will be evaluated using the Student's “t” or its nonparametric Mann–Whitney alternative.
Functional data. The differences in LVEF, LVMI, in the items of the quality of life questionnaire and in the calculation of the efficiency (in each branch of the study) will be evaluated using the Student's “t” test or its non-parametric alternative, the test of Mann–Whitney. To assess the difference in the existence of pericardial effusion, the Chi-square test or the Pearson test will be performed if the observed frequency distribution is not met.
Security controlsDuring follow-up, and especially in the experimental group, attention will be paid to volume overload, hyperkalemia and metabolic acidosis, as advised in usual clinical practice. The monthly bioimpedance in patients in progressive HD, and quarterly in the control group, will help to calculate of dry weight and to rule out overhydration. For the control of potassium and metabolic acidosis, researchers can perform a control of both parameters in the inter-monthly period.
The trial will be carried out in accordance with its protocol,22 with the guidelines of good clinical practice and with the applicable legal requirements in each country with participating centers. The confidentiality of the data will be carried out in accordance with Organic Law 15/1999 on the Protection of Personal Data and Royal Decree 1720/2007.
DiscussionThe transition from stage 5 not dependent of dialysis to the RRT is a crucial moment, both for the patient and for the nephrologist. You must choose, among others, 3 issues: when and how to start the RRT and the amount of extrarenal clearance that we must provide. Despite the absence of controlled studies to support it, there has been a tendency of early initiation of TRS.1 Thus, in the USA, more than 50% of the patients currently start with a KrU>3ml/min/1.73m2, without any evidence that this strategy had reduced morbidity and mortality.10
The initiation of progressive dialysis, defined as the gradual increase in dose of dialysis as RKF decreases, aims to maintain constant total solute clearance (Kr and Kd). In peritoneal dialysis, a progressive dialysis was already proposed in its first guidelines on adequacy,26 and is currently strongly implemented. Thus, in some countries 30% of patients start with one or two exchanges/day, or with 3 or 4 sessions/week of automated DP,27 and this happens despite the fact that studies on incremental PD are limited, with a low number of patients, non-randomized and from a single center.27
Progressive or incremental HD has also gained some importance in recent years. It has been carried out without economic purposes and has shown encouraging results in the maintenance of RKF, and with a survival similar to conventional HD.10–13 In fact, guide 3.2 of the KDOQI1 allows to reduce the weekly dose in patients with a KrU greater than 3ml/min/1.73m2. In these cases, the objective is continuous clearance of 2.3 volume weekly, expressed in stdKt/V terms, or an EKRU of 12-KrUml/min, both corrected for a volume of 35L.2,22 These recommendations are based on the strong correlation between RKF and survival,9 and the contribution of RKF to volume control and to the elimination of protein bound solutes via tubular secretion.28,29 It should be remembered that the protein bound solutes are poorly dialyzed with current techniques, even if frequency is increased.29,30
The studies published on incremental HD are observational, and their results should be taken with caution. In most studies patients were started with 2 weekly sessions.10–12 Presently there is not enough evidence to determine which regimen of dose or frequency, should receive incident patients in HD with RKF.
Based on previous experiences22,31 and according to some authors19,21 in DiPPI, we have proposed starting with a single weekly session and increasing to 2 and from 2 to 3 as the RKF declines. Although it seems daring, it is more logical to move gradually from stage 5 noD to stage 5HD. We hope to obtain the same survival and the same complication rate at 2 years. If this starting modality is confirmed to be as effective and safe, it will reduce the number of sessions i many incident patients. Thus, if one of every 4 HD patients in Spain initiate dialysis progressively, 76,000 sessions would be “avoided”, with their respective trips, and costs would decrease by more than 21 million euros per year.
The methodological design was carefully considered. In principle, an observational cohort design was chosen, controlling the selection bias by propensity score matching. This method must have enough variables to avoid bias, which implies a large control group to be able to find paired patients. But this does not eliminate the “residual confounding factors”, a threat in any observational study. A randomized controlled study has a minimal bias and provides the highest level of evidence, although it presents notable difficulties: lower power, selection of patients that produces randomization (may not be representative of the population in HD), or imbalances between both groups in some key variable. We believe that the sample calculation and randomization by blocks have minimized these drawbacks and will allow to respond the proposed hypothesis. It is not masked due the obvious difficulty of masking the sessions.
Possibly DiPPI is as necessary as previous studies such as HEMO,32 IDEAL33 or those resulting from of the FHN.4–6 The results of the DiPPI study will have the same importance as those mentioned. Buts as in the case of a non-commercial study, there is no funding for the inclusion of patients. Avoiding underdialysis is as important goal as overdializing, and this clinical trial will try to show whether there is a difference between progressive HD and fixed-dose HD 3 times a week in incident patients. The potential benefits and economic savings is a sufficient reason to carry out an effort by everyone. If you are interested in this topic or you value the possibility of participating in the study, we will provide you with all the necessary information.
AbbreviationsCRDe: electronic data collection notebook; DiPPI: incremental dialysis in incident patients (acronym of the study); CKD: chronic kidney disease; LVEF: left ventricular ejection fraction; RKF: residual kidney function; HD: hemodialysis; GFR: glomerular filtration rate; LVMI: left ventricular mass index; IRE: index of resistance to erythropoietin, Kd: dialytic clearance; KDOQI: Kidney Disease Outcomes Quality Initiative; KDQOL’36: Kidney Disease and Quality of Life; Kr: renal clearance; KrU: renal clearance of urea; stdKt/V: standard Kt/V; RRT: renal replacement therapy.
Ethics committee and endorsementsFavorable evaluation of the Ethics and Research Committee of the Hospital Complex of Cáceres. Endorsed by the Ministry of Health and Social Policies of the Junta de Extremadura and by the Spanish Society of Nephrology. Sponsored by the Foundation for Training and Research of Health Professionals of Extremadura (FundeSalud), under the Ministry of Health of the Junta de Extremadura.
Contribution of the authorsAll the authors have actively contributed to the design of the study, its methodology and the writing of the manuscript.
FundingCurrently, the project has an financial aid provided by Fundación Liberbank of 10,000 net euros, conveyed through FundeSalud.
Conflict of interestsNone of the authors stated that they had a conflict of interest.
To doctors J.L. Teruel and F. Maduell for their inestimable collaboration in the methodology and design of the study.
Please cite this article as: Suárez MA, García-Cabrera E, Gascón A, López F, Torregrosae E, García GE, et al. Justificación y diseño de DiPPI: un ensayo controlado aleatorizado para evaluar la seguridad y la efectividad de la hemodiálisis progresiva en pacientes incidentes. Nefrologia. 2018;38:630–638.