To the Editor,
Rhabdomyolysis is a syndrome with multiple causes and whose aetiology is unknown in many cases. Hyponatraemia is a rare cause of rhabdomyolysis and can go unnoticed if not suspected. In this instance, the rhabdomyolysis was related to hyponatraemia secondary to symptoms of nausea and vomiting.
Rhabdomyolysis is characterised by skeletal muscle injury, alterations in cell membrane integrity and release of intracellular products into the blood. This potentially lethal condition becomes worse with acute renal failure (ARF) in 4%-30% of patients, and causes 7%-10% of all ARF cases.1,2
There are multiple causes for rhabdomyolysis, which can be grouped as: 1) direct muscular trauma; 2) excessive muscular activity; 3) hereditary enzymatic defects (McArdle’s disease); 4) less evident causes, such as drugs (antipsychotic, antidepressive and lipid-lowering agents, cyclosporin, etc.), toxic agents, infections, autoimmune , endocrines (hypothyroidism, hyperaldosteronism, ketoacidosis) and electrolytic disorders.2,3 The pathophysiology for many of these processes converge into one final path that compromises the adenosine triphosphate (ATP) synthesis and the functioning of Na+/K+ and Na+/Ca++ pumps. It results in an increase of permeability to Na+ and an increase in intracellular Ca++, which starts an enzymatic activation and cell death process.3-5
Rhabdomyolysis is related to hyponatraemia/hypernatraemia, severe hypopotassaemia and hypophosphataemia which affect the membrane homeostasis and cell integrity. We present a clinical case of rhabdomyolysis with severe hyponatraemia.
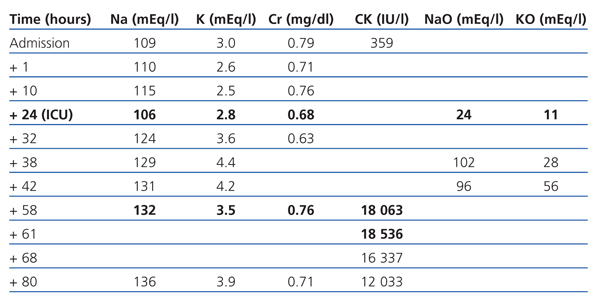
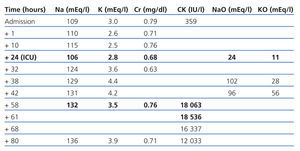
The patient is a 74-year-old female, with hypertension and dyslipidaemia. She was undergoing treatment with omeprazole, fluvastatin and tramadol. She presented with liquid diarrhoea and intractable vomiting, which had lasted a week. She was treated as an outpatient with oral fluid therapy. Her level of consciousness was reduced and she presented with a tonic-clonic seizure and sphincter relaxation. The emergency department reported that she had a Glasgow Coma Scale (GCS) score of 8 (M-5; E-2; V-1). The cranial computerised tomography (CT) showed former ischaemic involvement of the thalamus and internal capsule. Na: 109mEq/l, K: 3mEq/l, Cr: 0.79mg/dl and CK: 359IU/l. She was treated with saline solution at 2% and the natraemia was recovered up to 115mEq/l in the following 10 hours. Twelve hours after being admitted, her level of consciousness decreased again and she presented with: Na: 106mEq/l. Urine: NaO: 24mEq/l and KO: 11mEq/l. She was admitted to the intensive care unit (ICU) due to severe symptomatic hyponatraemia.
She received saline solution at 2% and at 0.9% for the first 24 hours. The natraemia was re-established, and she was restricted from liquid intake and diuretic treatment, showing daily negative balances of 1.5l-2l (Table 1). After 12 hours in the ICU, the patient was conscious and aware of her surroundings, although with a tendency to sleep, free of other organic failures and with Na+ in a non-critical range. She had CK: 18 063IU/l on the third day. After 4 days in intensive care, she was transferred to the medical ward.
Severe hyponatraemia is one of the most uncommon causes of rhabdomyolysis related to electrolyte alterations. This relationship between hyponatraemia and rhabdomyolysis has been described in psychiatric patients being treated with antipsychotic drugs and psychogenic polydipsia as a triggering factor.6-10 It is difficult to establish a causal relationship, given that both the neuroleptic malignant syndrome11,12 and hyponatraemia could be the cause of muscular necrosis. Our patient did not have any history of psychiatric problems or previous treatment for central nervous system disorders. Hyponatraemia is presented with gastroenteritis lasting several days, with no signs of dehydration of mucous, hypotension or hypovolaemia, and with preserved renal function and diuresis. NaO is higher than 10mEq/l and osmolarity is higher than that of plasma. Without being able to rule out a poorly recovered hypovolaemic hyponatraemia, we suspected a normovolaemic hyponatraemia secondary to the ‘physiological’ release of the anti-diuretic hormone caused by nausea and vomiting.13 Another possible cause of rhabdomyolysis are convulsions. Our patient presented with a single generalised tonic-clonic seizure secondary to severe hyponatraemia. The increase in CK during convulsive episodes is not very significant, except for refractory epilepsy. Statins, especially those associated with other lipid-lowering agents, may cause complications such as myalgia and muscular weakness, with an increase in CK (0.1%-0.5%), and in exceptional cases severe rhabdomyolysis (0.04%-0.2%).2,12 As a causal mechanism, a reduction in the co-enzyme Q levels and ATP production is considered, with an alteration in the cell membrane due to the reduced cholesterol synthesis. The patient continued treatment with fluvastatin, which is the drug that is least likely to produce rhabdomyolysis.14 This could explain the minimal increase in CK upon admission, although we could not rule out that it was a sign of hyponatraemia. In either case, treatment was withdrawn, and the increasing CK level figures do not support this causal relationship.
We relate rhabdomyolysis with severe hyponatraemia. Different hypotheses could explain the physiopathology of this relationship. The first suggest that the muscular injury is a consequence of a failure in cell volume regulation and ionic balance. Acute hyponatraemia provokes an aqueous intoxication secondary to hypo-osmolarity of extracellular fluid. The osmolar balance is normalised after several hours due to K+ leaving the cell, causing the membrane potential to decrease and altering the cellular metabolism.6 Another possible mechanism is an alteration in the cell membrane’s Na+/Ca++ pump. The hyponatraemia reduces the gradient of Na+ input within the muscle cell and reduces the Ca++ output. This increase in intracellular Ca++ started an enzymatic activation and cellular death process.5 A third hypothesis suggests that Na+ regulation is the cause of rhabdomyolysis, and the compensatory mechanisms developed by the cell are not capable of maintaining the homeostasis in the regulation of cellular volume given that Na+ is corrected too quickly.6-9 Replacing more than 1mEq/1 hour of Na during the first 12 hours is not recommended, especially no more than 12mEq/24 hours. This mechanism would explain why the enzymatic curve shows a delayed peak (48-72 hours from hyponatraemia onset). The enzymatic peak for our patient was 18 000IU/l on the third day from the onset of hyponatraemia, but only 24 hours after she experienced a rapid correction of Na (18mEq) in only eight hours.
In summary, we consider hyponatraemia, and a too rapid correction of it, as often being the cause of rhabdomyolysis that could go unnoticed. Knowing the severity of the rhabdomyolysis-associated complications, mainly those regarding ARF, it is necessary to prevent such eventuality and to treat it correctly and early. In our case there were no added complications, probably because the patient had an abundant diuresis throughout the evolution.
Table 1. Evolution of biochemical parameters over time