Objective: To update the 2010 recommendations on the evaluation and management of renal disease in HIV-infected patients. Methods: This document was approved by a panel of experts from the AIDS Working Group (GESIDA) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), the Spanish Society of Nephrology (S.E.N.), and the Spanish Society of Clinical Chemistry and Molecular Pathology (SEQC). The quality of evidence and the level of recommendation were evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system. Results: The basic renal work-up should include measurements of serum creatinine, estimated glomerular filtration rate by CKD-EPI, Urine protein-to-creatinine ratio, and urinary sediment. Tubular function tests should include determination of serum phosphate levels and urine dipstick for glucosuria. In the absence of abnormal values, renal screening should be performed annually. In patients treated with tenofovir or with risk factors for chronic kidney disease (CKD), more frequent renal screening is recommended. In order to prevent disease progression, potentially nephrotoxic antiretroviral drugs are not recommended in patients with CKD or risk factors for CKD. The document advises on the optimal time for referral of a patient to the nephrologist and provides indications for renal biopsy. The indications for and evaluation and management of dialysis and renal transplantation are also addressed. Conclusions: Renal function should be monitored in all HIV-infected patients. The information provided in this document should enable clinicians to optimize the evaluation and management of HIV-infected patients with renal disease.
* The authors José L. Górriz, Félix Gutiérrez, Joan C. Trullas and José M. Miró contributed equally to this document
1. INTRODUCTION
Since the end of the 1990s there has been a progressive change in the natural history of infection with the human immunodeficiency virus (HIV) with a sustained decrease in the incidence of the acquired immunodeficiency syndrome (AIDS) and related mortality1. At present, most patients with good adherence to treatment have a long life expectancy2 and those who have received antiretroviral therapy (ART) for at least six years and have achieved a CD4+ lymphocyte level greater than 500 cells/ml have an estimated mortality that is similar to that of the general population3. Despite this decrease in HIV-related mortality, there has been an increase in the proportion of deaths by other causes. Furthermore, greater longevity resulted in an increase in comorbidity related to chronic conditions such as diabetes mellitus (DM), high blood pressure (HBP), dyslipidemia and heart diseases, amongst others4.
In recent years, various cohort studies have highlighted the importance of renal disease as a cause of morbidity and mortality in HIV-infected patients5,6. In addition to nephropathies specifically associated with HIV or infection with the hepatitis C virus (HCV), increased patient longevity, a greater prevalence of metabolic abnormalities and the accumulation of vascular risk may be favouring the development of chronic kidney disease (CKD) in the HIV-infected population. The recognition that certain antiretroviral drugs may cause renal damage in some patients is an additional concern.
The long-term consequences of chronic renal dysfunction in HIV-infected patients are not yet well-known. In the general population, CKD has a considerable multiple-organ impact, which could have special implications in patients who are also infected with HIV. Furthermore, a decreased glomerular filtration rate (GFR) makes it necessary to adjust the dose of many antiretroviral drugs and that of other medications necessary for treating associated comorbidities.
The current clinical guidelines recommend introducing ART when HIV-associated nephropathy (HIVAN) has been diagnosed, regardless of the patient’s virological and immunological condition7,8.
The incidence and prevalence of CKD in HIV-infected patients are difficult to determine and vary according to the type of study, the geographic region and the criteria used to define renal involvement (GFR estimation, an increased serum creatinine concentration, the presence of proteinuria, etc.)9. Studies carried out in the scope of the European Union state that the prevalence of HIV infection in dialysis patients is low, at around 0.5%10-12.
The objective of this document is to provide recommendations, based on scientific evidence, on the prevention, diagnosis and management of renal disease in HIV-infected patients, updating the recommendations for the evaluation and treatment of renal disorders published in April 2009 by the AIDS Study Group (GESIDA) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) and the Secretariat of the Spanish National AIDS Plan (PNS)13. In these recommendations, strategies were agreed for monitoring, controlling and preventing renal damage in HIV-infected patients.
This document is intended for all professionals of different specialities who treat HIV-infected patients.
2. METHODOLOGY
This joint document is the result of incorporating the updated version of the previous GESIDA and PNS document13 to a working document prepared by an ad hoc group. The original documents were written by experts in Infectious Diseases and HIV, Nephrology specialists and Clinical Biochemistry specialists, selected by the governing boards of GESIDA of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), the Spanish Society of Nephrology (S.E.N.) and the Spanish Society of Clinical Biochemistry and Molecular Pathology (SEQC). Each panel member issued a conflict of interest declaration, which is included at the end of this document. Three coordinators were appointed to incorporate the two documents and write the joint document (one by GESIDA, another by S.E.N. and the other by SEIMC), as well as a managing editor. The coordinators prepared the list of topics in this document, which was approved by all members of the panel. The chapters relating to HIV were written and revised by GESIDA experts, those relating to renal pathology were written and revised by S.E.N. experts and aspects regarding laboratory tests for the study of renal function were written and revised by SEQC experts.
The joint document was revised and approved by the coordinators and all authors and submitted for an external review; it was displayed on the websites of the promoting organizations (GESIDA, S.E.N. and SEQC) for a period of time in order that the professionals for whom it is intended and any interested person could suggest adjustments or changes, which were considered by the group and possibly included.
In this document, the grade of recommendation and quality of the supporting evidence are based on the GRADE (Grading of Recommendations Assessment, Development and Evaluation)14-19 system, with the definitions being summarized in a previous document20. In situations in which the recommendation could not be classified, we used the term “Recommendation based on consensus”. GESIDA-SEIMC, S.E.N. and SEQC undertake to update these guidelines at a future date in accordance with the advancement of knowledge in this subject area.
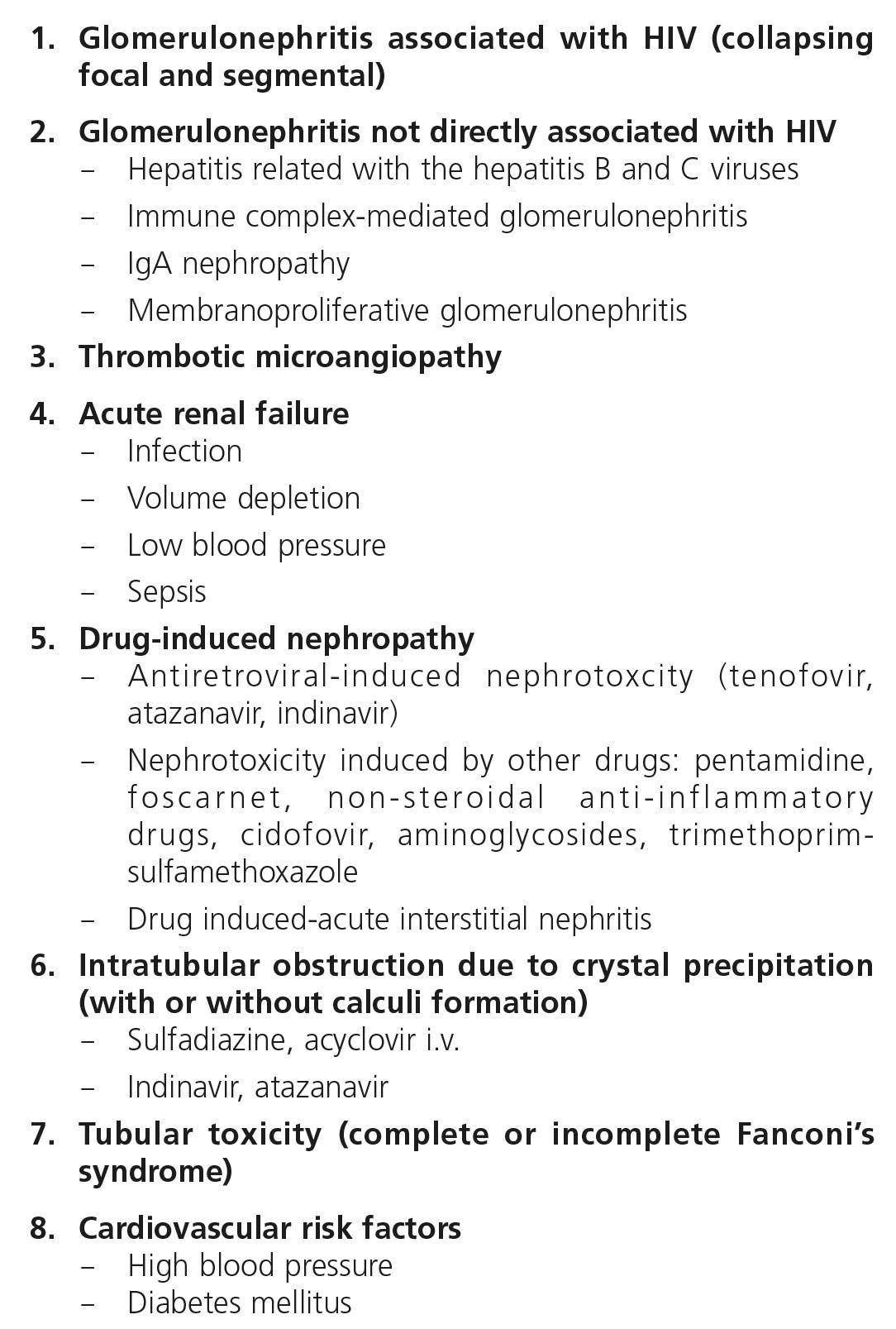
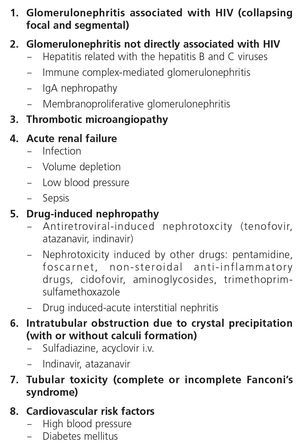
3. CLASSIFICATION OF RENAL DISEASES IN HIV-INFECTED PATIENTS
3.1. Types of renal disease in HIV-infected patients
Renal disease in HIV-infected patients may present as acute or chronic forms (Table 1).
3.1.1. Acute renal failure
This is characterized by a rapid deterioration of renal function, resulting in difficulty to eliminate waste products, water and electrolytes.
We define acute renal damage or failure as an increase in serum creatinine concentration equal to or greater than 0.3mg/dl (26.5mmol/l) in 48 hours, or an increase equal to or greater than 50% (x 1.5) of its baseline value in a period of 7 days, or diuresis lower than 0.5ml/kg/h in 6 hours21.
Acute renal failure (ARF) is present in 6% of patients hospitalized with HIV infection and it is associated with a mortality rate of 27%22. As such, it is recommended to closely monitor renal function during hospitalization, particularly from serious illnesses23,24. In outpatients, prevalence increases to 10%, with an incidence of 5.9 episodes per 100 patients-year25, and is secondary to drugs in 33% of cases. In general, ARF is usually reversible and the factors that favour its onset are similar to those in the general population: advanced age, pre-existing diseases, CKD, sepsis, severe systemic diseases, acute and chronic infections and exposure to nephrotoxic agents, including antiretroviral drugs and other drugs used for treating opportunistic infections22,26. Immunodeficiency has also been considered to be an important risk factor for ARF27. There may occasionally be transitory renal function deterioration, favoured by extrarenal factors such as dehydration, vomiting, diarrhoea, low blood pressure, non-steroidal anti-inflammatory drugs (NSAIDs) or the combination of several of these factors and if the situation persists, it may cause the onset of CKD, favoured by the use of potentially nephrotoxic antiretroviral drugs28,29.
3.1.2. Chronic kidney disease
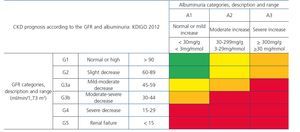
This is defined as a decrease in the GFR (<60ml/min/1.73m2) or renal damage (proteinuria, albuminuria, histological abnormalities in the biopsy, in the urinary sediment or in imaging techniques) that persists for more than three months30,31.
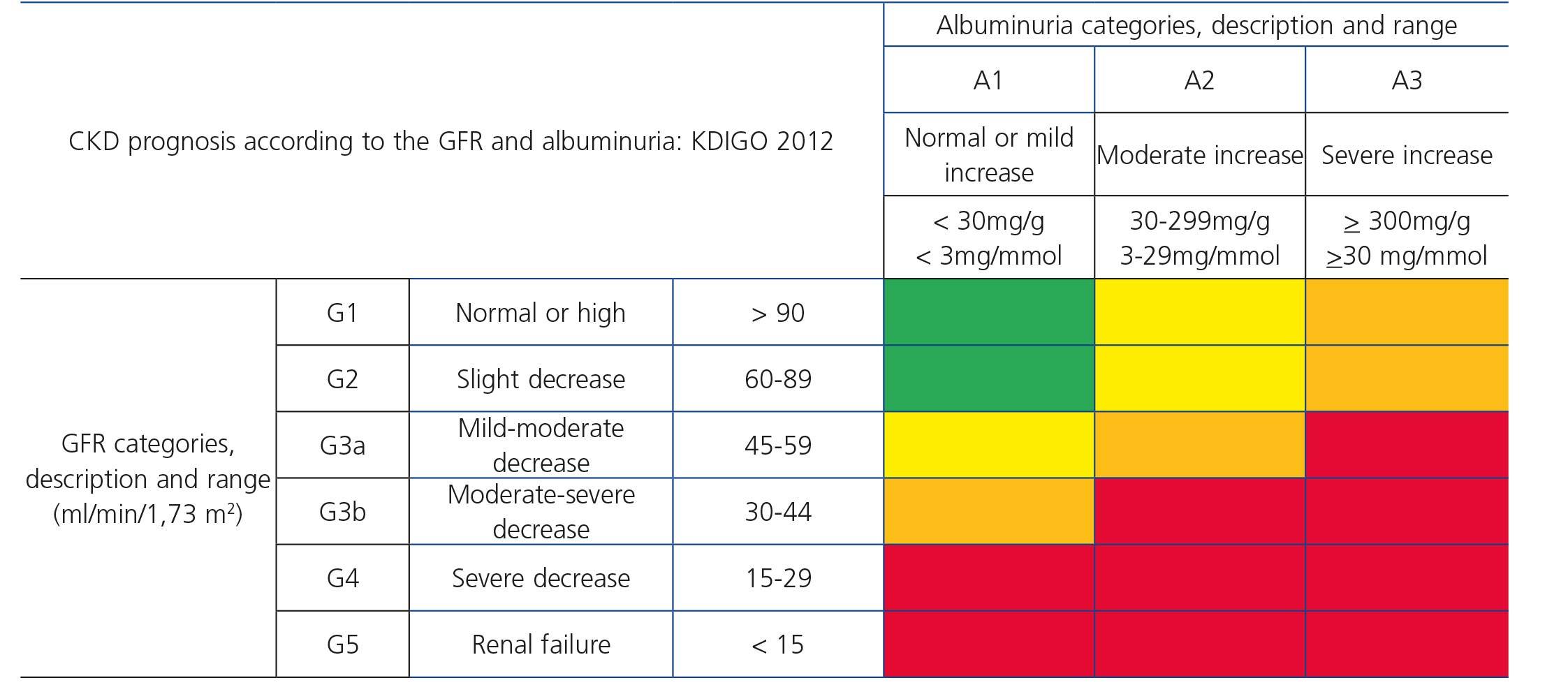
Recently, from the results of different studies that include healthy individuals (or those without CKD), individuals at risk of developing CKD and patients with CKD, the international organization KDIGO (Kidney Disease Improving Global Outcomes)32 (Table 2) has established a new CKD prognosis classification based on GFR and albuminuria values. This classification is divided into six risk categories according to the GFR, which are complemented by three risk categories according to the urinary albumin/creatinine ratio (ACR) value from data of a meta-analysis of general population cohorts. The risk shown in the table with different colours has been calculated from data of a meta-analysis of general population cohorts and includes five events: overall mortality rate, cardiovascular mortality, renal failure treated with dialysis or transplantation, acute renal failure (ARF) and progression of renal disease32.
CKD prevalence in HIV-infected patients depends on the study populations and is higher in Black individuals and in areas with less access to treatment.
Around 7.1% of HIV-infected patients have an estimated glomerular filtration rate (eGFR)31 <60ml/min/1.73m2 and in other studies the prevalence of albuminuria has varied between 11% and 15.5%33.
Risk factors for the onset of CKD include HBP, DM, advanced age, genetic factors, Black race, a family history of CKD, coinfection with the hepatitis B virus (HBV) or HCV, a low CD4 nadir, a high HIV viral load and the use of potentially nephrotoxic medication34. It must be noted that as the life expectancy of the HIV-infected population increases, traditional cardiovascular risk (CVR) factors such as HBP and DM become more prominent in favouring the onset of CKD.
The incidence and prevalence of CKD in HIV-infected patients are difficult to determine and vary according to the type of study, the geographic region and the criteria used to define renal involvement (GFR estimation, an increased serum creatinine concentration, the presence of proteinuria, etc.)9. Studies carried out in the scope of the European Union state that the prevalence of HIV infection in dialysis patients is low, at around 0.5% (0.54 in Spain, 61% coinfected with HCV in 2006)10-12. Many of these patients may have access to a renal transplant.
The reasons for the introduction of renal replacement therapy have changed in recent years. In the early days of AIDS, HIVAN and nephropathies related to HBV and HCV were the most common causes. There has been an increase in causes related to the use of drugs and associated comorbidities, mainly DM and HBP35,36. The widespread use of ART has altered the clinical course of renal disease in HIV-infected patients37.
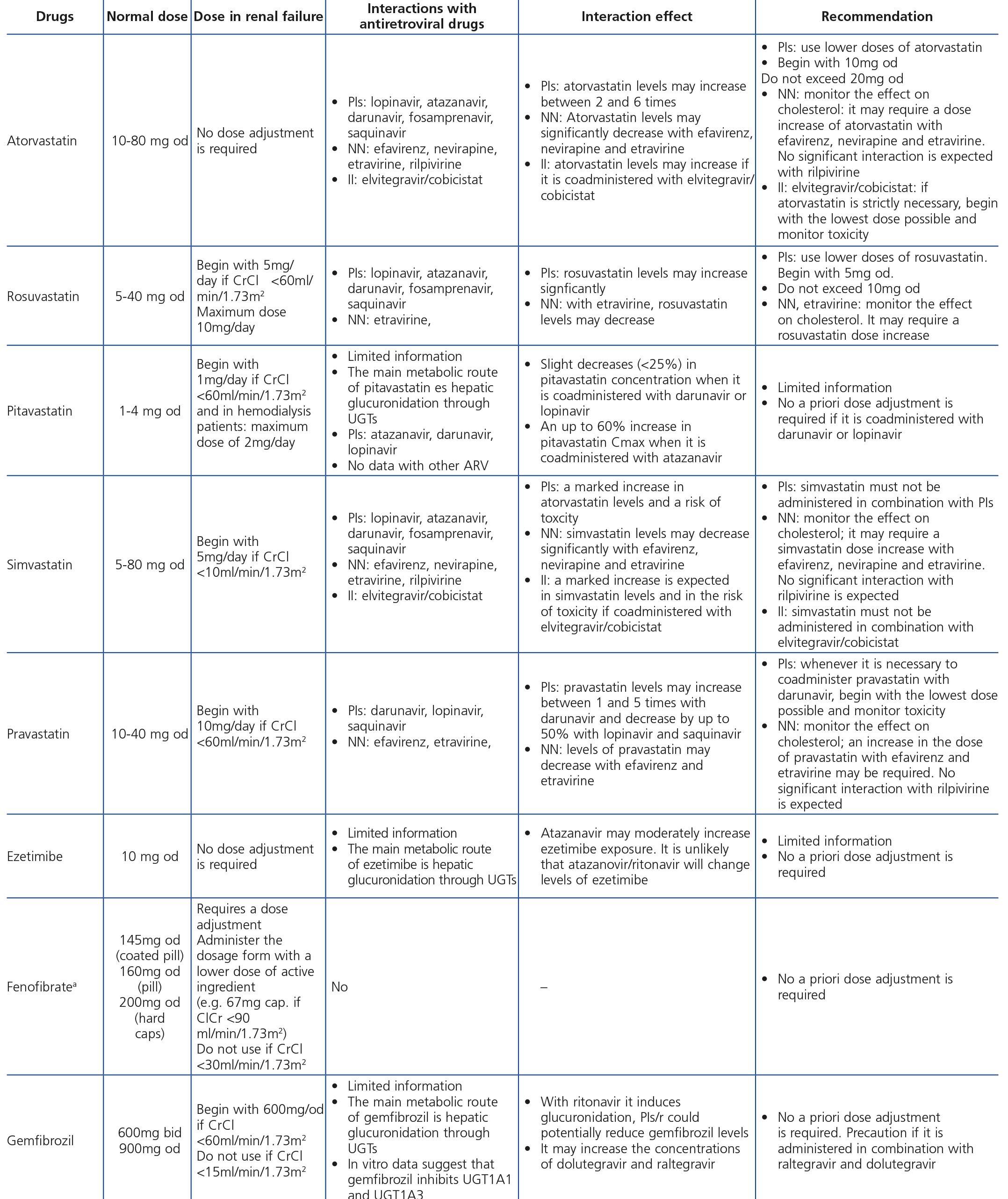
3.1.3. Antiretroviral drug-induced nephrotoxicity
ART-associated nephrotoxicity is uncommon, although it is expected to be higher with an increase in the life expectancy of HIV-infected patients and the presence of comorbidities. The aetiopathogeny of renal toxicity from antiretroviral drugs is mainly due to functional abnormalities of transport proteins in epithelial cells of the proximal convoluted tubule, mitochondrial toxicity, vascular lesions and crystal precipitation at the tubular level. In most cases, the drugs involved in renal toxicity are nucleoside reverse-transcriptase inhibitors (NRTIs) and in particular tenofovir (TDF) and protease inhibitors (PIs). Some PIs (atazanavir [ATV] and lopinavir [LPV]) have been associated with a higher risk of a decrease in the eGFR, although this clinical effect is controversial and may be due to the interaction of ritonavir (RTV) with TDF when they are administered simultaneously38-44.
With regard to NRTIs, TDF is the main drug involved in nephrotoxicity and its excretion is mediated by the action of transport proteins, which help eliminate the drug to the tubular lumen so it appears in urine. Blocking these proteins may favour the accumulation of the drug in the renal tubular cells and nephrotoxicity45,46. TDF-induced toxicity may cause proximal tubular dysfunction and acute tubular necrosis, with the possibility of progression to CKD. Proximal tubular dysfunction or Fanconi’s syndrome includes phosphaturia, glucosuria with normoglycemia, renal tubular acidosis with normal anion gap, aminoaciduria, tubular proteinuria and medium-long-term renal failure. The earliest signs are phosphaturia, metabolic acidosis and glucosuria. TDF toxicity is usually reversible when the drug is withdrawn, although recovery may not be complete47. With regard to PIs (mainly indinavir [IDV] and ATV), renal toxicity is caused by the low solubility of these drugs in urine in certain pH conditions, causing crystalluria and tubular obstruction. Crystal-induced toxicity is more common with IDV, currently very rarely used, which is soluble in acidic urine, but relatively insoluble in alkaline urine, with the latter situation favouring the formation of crystals48. Crystal precipitation in urine was also reported with darunavir (DRV)49.
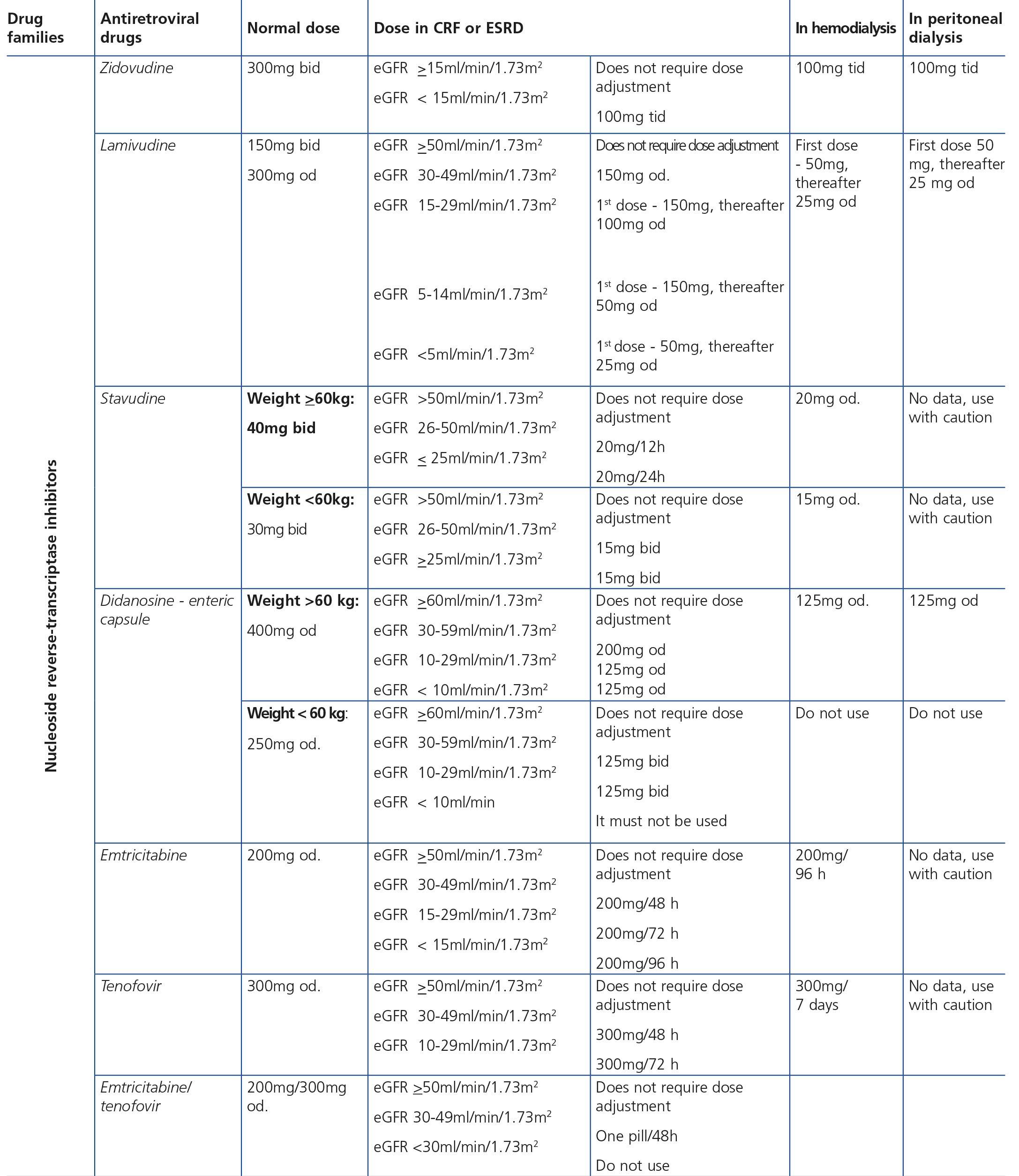
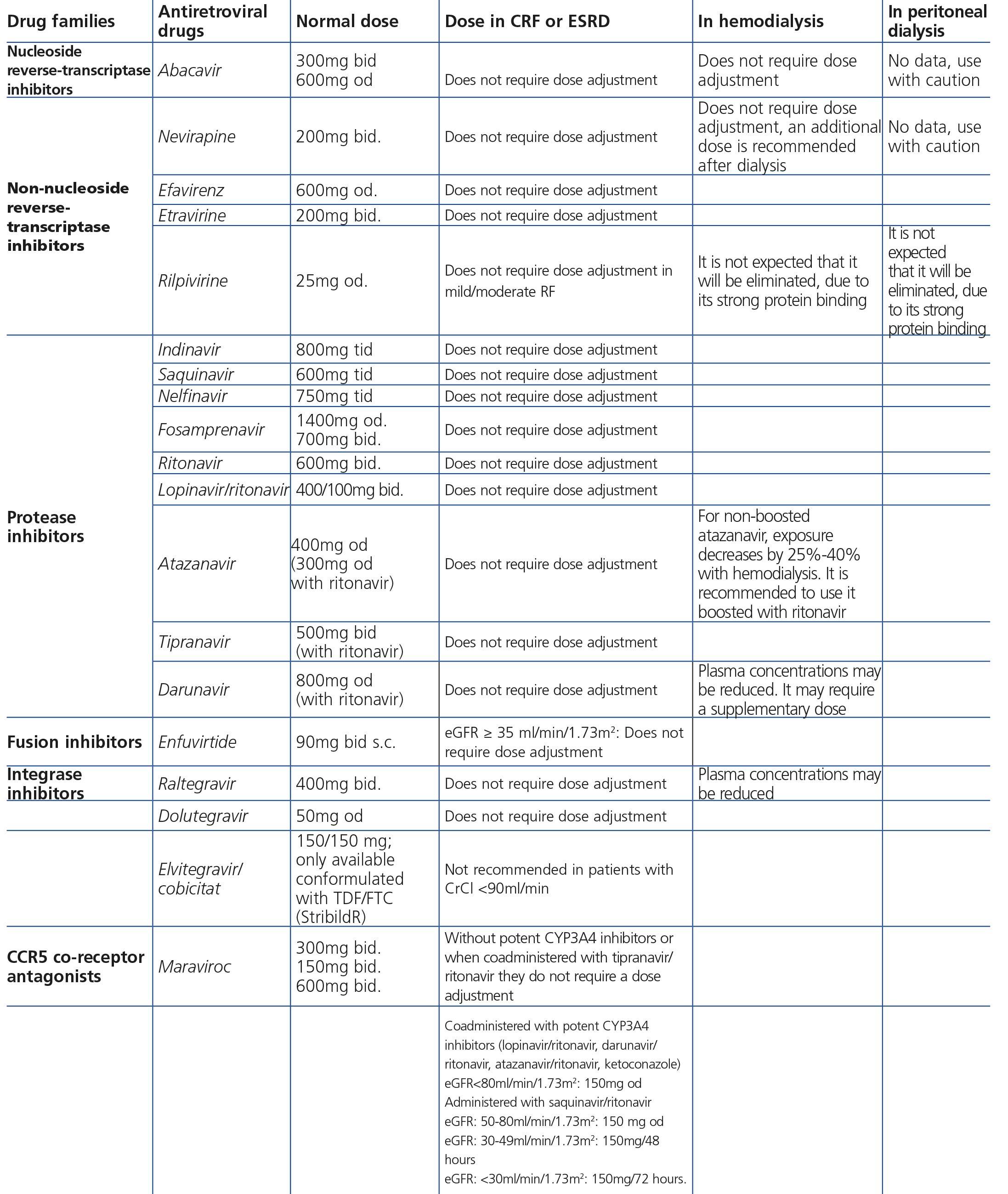
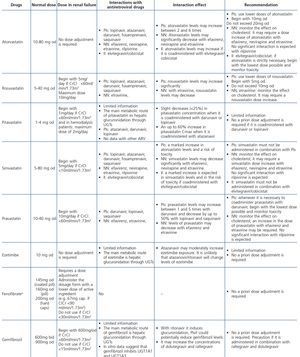
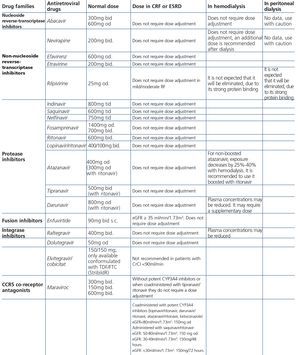
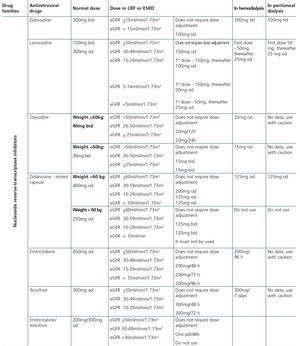
Some new antiretroviral drugs interfere with active tubular creatinine secretion. Interactions with creatinine transport have been identified with creatinine transport with rilpivirine (RPV)50, dolutegravir (DTG)51 and the pharmacokinetic booster cobicistat (COBI)52,53. While RPV and DTG mainly inhibit the renal organic cation transporter type 2 (OCT2)50,51, COBI mainly inhibits the multidrug and toxin extrusion protein transporter type 1 (MATE1)53. This interaction with the transporters involved in the tubular secretion of creatinine may cause slight increases in serum creatinine and a resulting decrease in the eGFR, which do not reflect true decreases in the GFR51,54. Although in most clinical trials carried out to date there have been no signs that the co-administration of these drugs significantly increases the risk of developing tubular toxicity with TDF, the information available on the renal safety of these combinations is still limited and there may be differences between the different drugs. In one clinical trial that developed the coformulation of tenofovir/emtricitabine/cobicistat/elvitegravir (TDF/FTC/COBI/EVG), study GS-236-0103, in the analysis after 96 weeks, 10 (1.4%) patients in the TDF/FTC/COBI/EVG group (n=701) and 2 (0.6%) in the comparison group, which received tenofovir/emtricitabine/atazanavir/ritonavir (TDF/FTC/ATV/r) (n=355), had to discontinue the regimen assigned due to an adverse renal reaction. Of these discontinuations, 7 in the TDF/FTC/COBI/EVG group and 1 in the TDF/FTC/ATV/r group occurred in the first 48 weeks55,56. The renal toxicity profile observed with TDF/FTC/COBI/EVG was similar to that previously reported with TDF and it mainly consisted of proximal tubulopathy, generally reversible with the withdrawal of the drug. Of the 4 patients with Fanconi’s syndrome in trial GS-236-0103, 2 had a baseline renal function disorder (estimated creatinine clearance [CrCl] <70ml/min)55,56. The antiretroviral drug adjustment in patients with different grades of renal dysfunction is described in detail i• Section 9.
3.1.4. Non-antiretroviral drug-induced nephrotoxicity
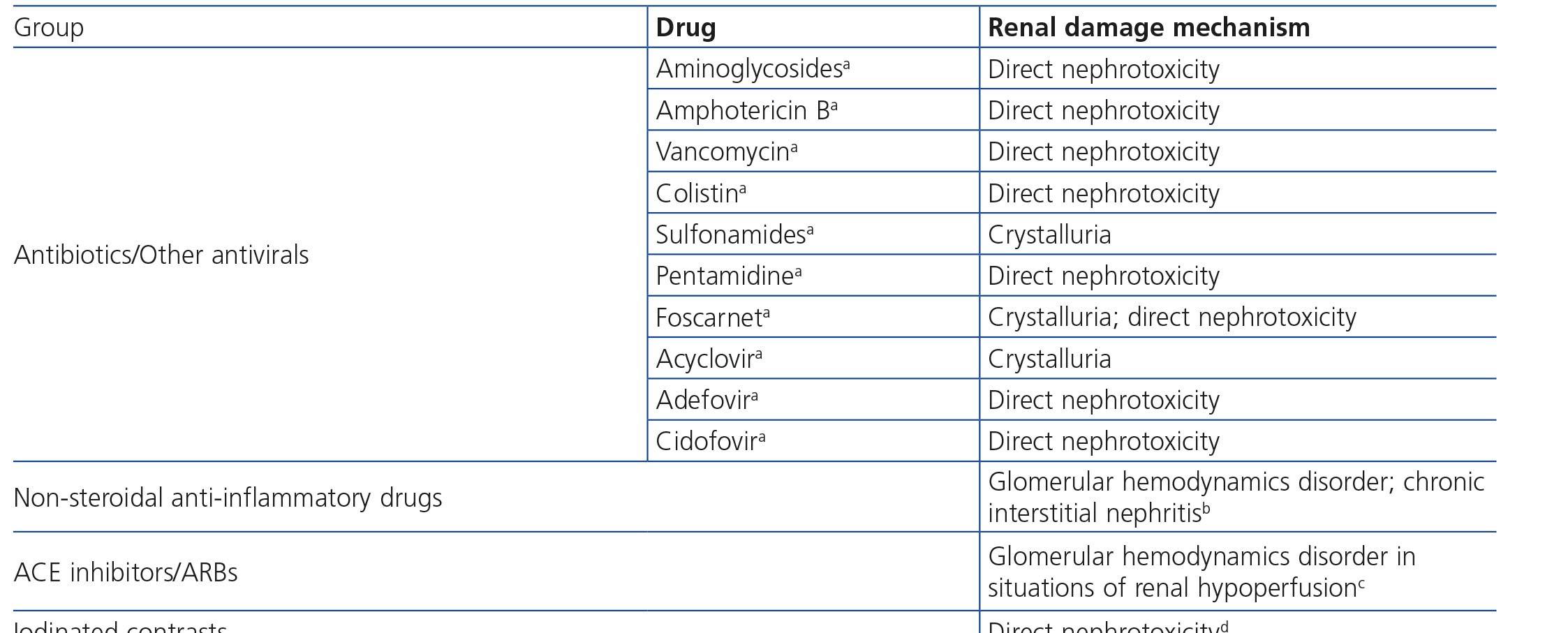
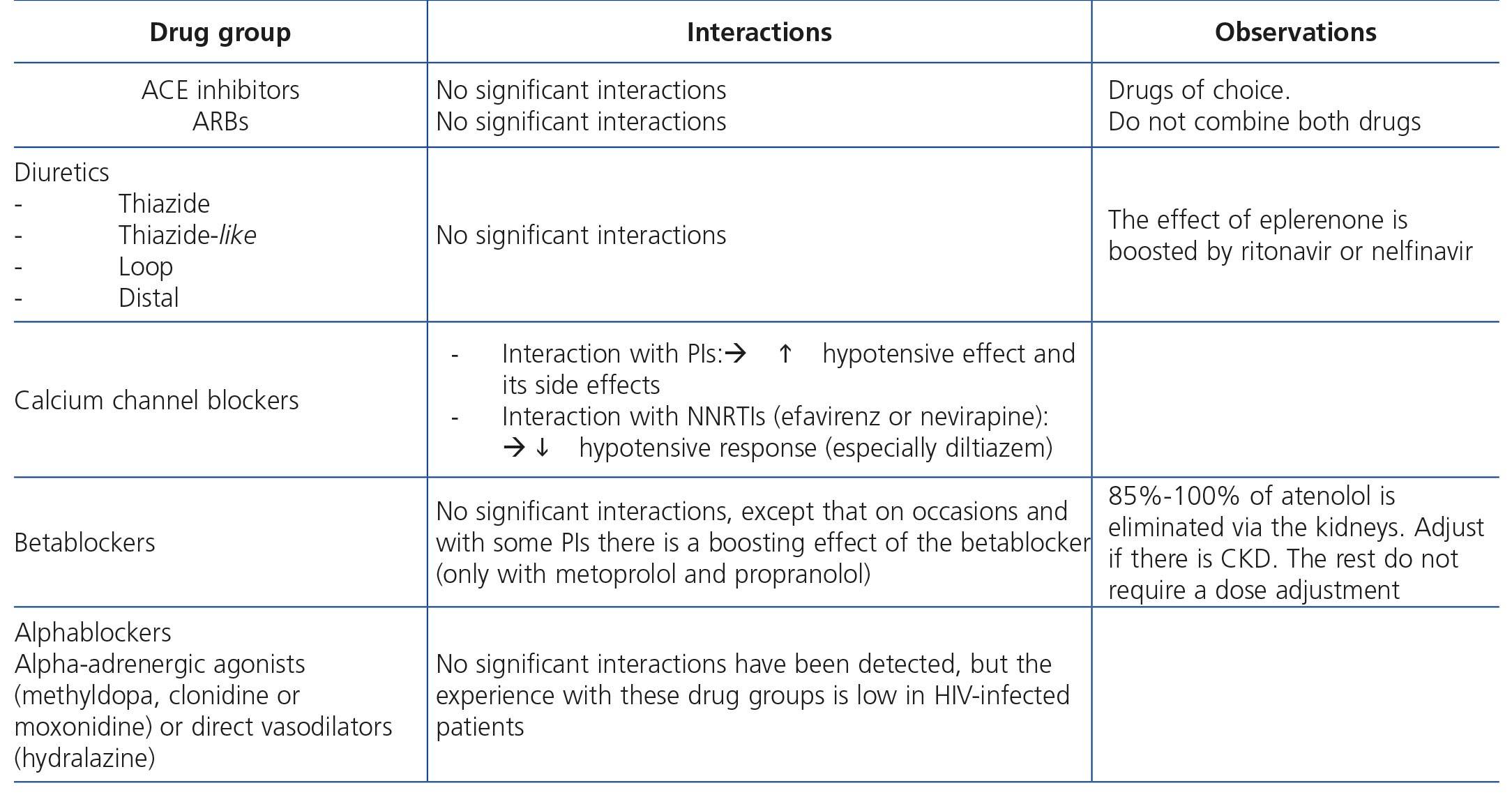
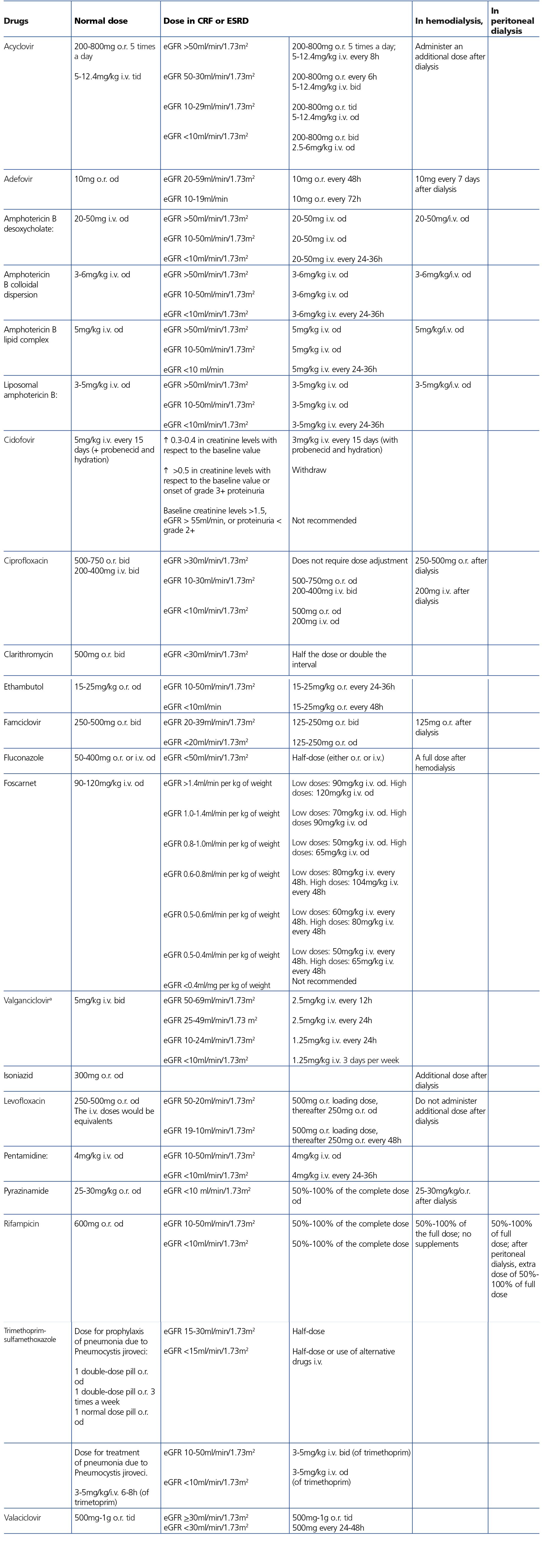
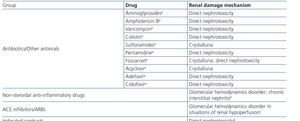
HIV-infected patients may, at some point during the clinical course of their condition, receive other potentially nephrotoxic drugs. Nephrotoxicity has several mechanisms including: direct nephrotoxicity (iodinated contrasts, aminoglycosides, amphotericin B, vancomycin, pentamidine, foscarnet), which must be avoided in patients with renal failure or for which a dose adjustment is required (acyclovir, ganciclovir), hemodynamic mechanisms (NSAIDs or angiotensin-converting-enzyme inhibitors [ACE inhibitors]/ angiotensin receptor blockers [ARBs] in certain situations such as decreased GFR), and crystalluria production. Table 3 summarises the potentially nephrotoxic non-antiretroviral medications and the renal damage mechanism.
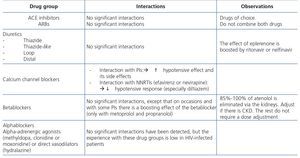
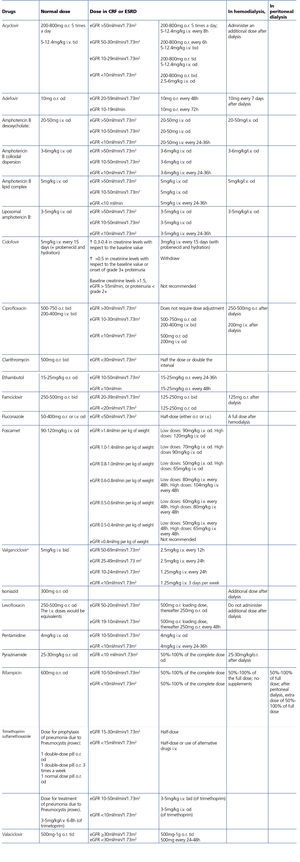
The administration of ACE inhibitors or ARBs may be associated with renal deterioration in certain clinical situations (see section 7.2.3.3.3). On the other hand, these drugs are particularly useful for cases of proteinuria or in treating HBP. Furthermore, NSAIDs may cause hemodynamic deterioration in patients with decreased renal function if they are administered over long periods of time or in combination with other drugs that may interfere in glomerular hemodynamics or potentially nephrotoxic drugs, such as TDF (see section 7.7.2). NSAIDs may be administered if it is strictly necessary in patients with normal renal function who do not simultaneously receive drugs that alter glomerular hemodynamics and over short periods of time. These drugs must be used with great caution (see section 7.2.3.3.3.) in patients with renal failure, their dose must be carefully adjusted and the evolution of renal function must be monitored closely during their use. I• Section 9, we will specifically address antiretroviral and non-antiretroviral drug adjustment according to the eGFR.
3.2. The most common renal diseases in HIV-infected patients
HIV-infected patients may develop various glomerular, vascular, tubulointerstitial and obstructive nephropathies, secondary to the virus itself, to administered drugs and/or to coinfection.
3.2.1. Glomerular and vascular nephropathies
The spectrum of glomerular pathology in HIV-infected patients depends on race, the control of HIV and the presence or not of accompanying infections, such as HCV. This has certainly changed in recent years with a lower incidence of HIVAN and a higher prevalence of classic focal and segmental hyalinosis being observed, associated with older age and a higher number of CVR factors57.
3.2.1.1. HIV-associated nephropathy
This type of glomerular involvement is the best characterized form. It is much more common in Black patients than in Caucasian patients (12:1)58,59. Although initial reports associated the onset of HIVAN with advanced stages of the HIV infection, it may also develop in asymptomatic patients60. The main manifestation of HIVAN is high proteinuria, generally greater than 2-3g/24h, and which frequently reaches nephrotic range (>3.5g/24h)58,61. Despite this, the clinical impact of proteinuria (edema, hypoalbuminemia, hyperlipidemia) is lower than in patients with other causes of nephrotic syndrome. The urinary sediment is rather inexpressive, although in many patients, insignificant microhematuria and leukocyturia are observed. Despite the characteristic tendency for Black patients with renal diseases to have HBP, the latter does not always accompany the HIVAN nephrotic syndrome. At ultrasound examination, kidney size is normal or even increased, and there is a marked and characteristic hyperechogenicity. The clinical course of HIVAN without ART is unfavourable, with a rapid development of renal failure requiring dialysis within the first year of diagnosis and with a high mortality rate62. Since the introduction of combined ART, clinical course has been improved63. Its histological substrate is collapsing focal glomerulosclerosis with intense tubulointerstitial involvement and dilation of renal tubules, which occasionally form authentic pseudocysts64. Renal immunofluorescence usually shows non-specific deposits of immunoglobin M (IgM) and C3. Immune complex deposits are not observed, which is important in the differential diagnosis. With regard to the pathogenesis, it is considered that there is direct involvement of HIV itself in the production of glomerular cell abnormalities65. Along with this, a recently identified genetic mutation (locus MYH9-APOL1), very common in African Americans, explains the association between HIVAN and the Black population66.
Although there have been no controlled clinical trials, data from observational studies suggest that ART reduces the risk of developing HIVAN and improves the prognosis of patients who have developed this nephropathy63,67-70.
Blocking the renin-angiotensin system with ACE inhibitors or ARBs induces an antiproteinuric and renoprotective effect in HIVAN patients, comparable with that observed in other nephropathies71, which may delay progression of renal failure. Some studies have shown a decrease in proteinuria and a tendency to slowing down the progression of renal damage in HIVAN patients treated with steroids. However, steroid treatment may induce major and common side effects, particularly in patients with more impaired immunity72.
Treatment of HIVAN includes ART, ACE inhibitors or ARBs and glucocorticoids. ACE inhibitors or ARBs are indicated if there is HBP or proteinuria71. Corticosteroids are not used routinely in these patients and although some minor clinical trials have shown their benefit, they must only be administered in patients who have progressive renal disease despite ART and ARB or ACE inhibitor use72. The histology in the biopsy may provide information about parameters that require treatment with steroids (for example, crescents).
3.2.1.2. Immune complex-mediated glomerulonephritis
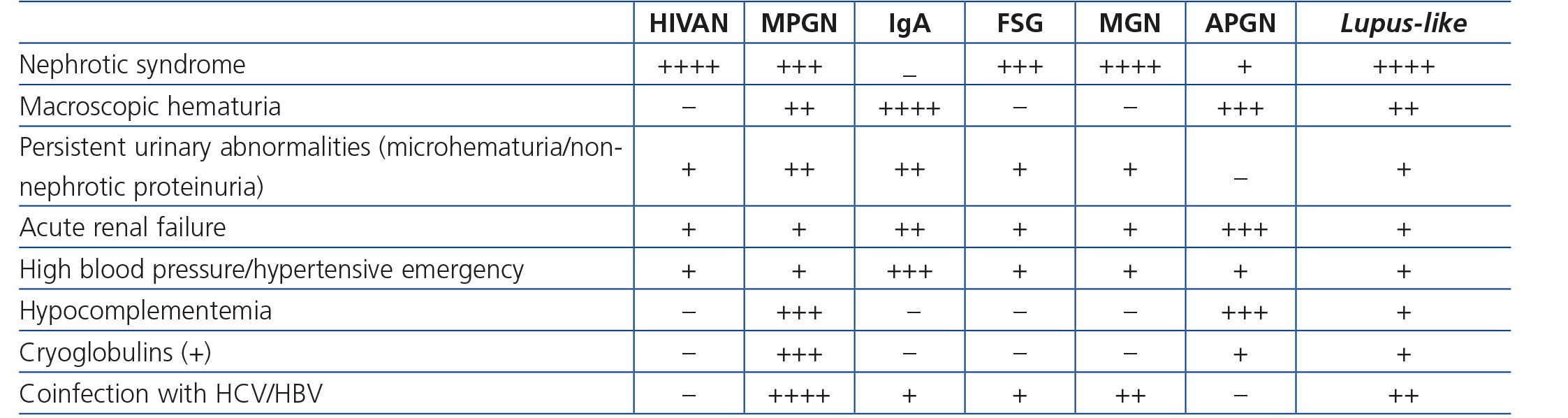
As well as HIVAN, HIV-infected patients have a higher incidence of other glomerulonephritis (GN), whose pathogenesis is generally attributed to glomerular immune complex deposits. The Black population is not predisposed to immune complex-induced GN as in the case of HIVAN (in fact, they are more prevalent in the Caucasian population) and these glomerulonephritis have mainly been observed in European countries73-75. The renal histology observed in these patients is extremely varied and includes proliferative (10%-80%), lupus-like and mixed proliferative and sclerotic forms. They are occasionally not directly associated with HIV infection but rather with other coinfections, such as that caused by HBV or HCV, related to a great variety of renal diseases such as membranoproliferative GN with cryoglobulinemia or membranous nephropathy. Nephropathy due to immunoglobulin (IgA) (IgA deposit-induced mesangial GN) is one of the most common immune complex-mediated nephropathies in HIV-infected patients in Europe.
Clinical manifestations of immune complex-mediated GN are usually very manifest (macroscopic hematuria, edema, ARF, severe HBP), although there are also cases of more subtle manifestations, which are diagnosed adventitiously (for example, non-nephrotic proteinuria, microhematuria or slowly progressive deterioration of renal function). In some cases of membranoproliferative GN, extrarenal manifestations of cryoglobulinemia are displayed in the clinical presentation, with purpura, digestive manifestations and even alveolar hemorrhage. In these patients, ARF with hematuria and proteinuria may occur, and renal biopsy shows, besides the typical lesions of membranoproliferative GN some cryoglobulin deposits in the lumens of glomerular capillaries. Cryoglobulins associated with HCV are generally mixed (IgG-IgM). Circulating cryoglobulins are detected in most cases, along with increased rheumatoid factor and decreased complement, particularly C4. This clinical-serological profile is very similar to that of membranoproliferative GN associated with HCV in patients without HIV infection. It is considered that these are GN pathogenically induced by HCV, without the concurrent presence of HIV playing a notable pathogenic role75-77.
Although clinical presentation may inform us about the type of GN (Table 4), for a definitive diagnosis, sometimes a renal biopsy is required for a definitive diagnosis. The indication of a biopsy must always be individualized, weighing up the risk of the procedure and the clinical benefits that its results may provide for the patient. This aspect is discussed i• Section 6.
The data available on the treatment of immune complex-mediated GN in HIV-infected patients are very limited. Information on the natural history is still very poor and it is not known whether it may be modified through therapies used in patients without HIV infection (steroids, immunosuppressants, calcineurin inhibitors). The effect of treatment with interferon (IFN) and ribavirin on the clinical course of HCV-associated nephropathies in HIV-infected patients is to a large extent unknown. Therapeutic recommendations are based on the experience with the treatment of HCV-associated membranoproliferative GN in monoinfected patients. There are series of cases in which an effective antiviral treatment (sustained clearance of the ribonucleic acid [RNA] of HCV in plasma) has been followed by an improvement in renal manifestations77,78. However, cryoglobulinemia may persist over long periods after clearance of the RNA of HCV in plasma and may be asymptomatic. Rituximab, an anti-CD20 monoclonal antibody, has caused a sustained improvement of renal manifestations in cases of membranoproliferative GN in monoinfected patients in whom a virological control of HCV had not been achieved.78
3.2.1.3. Diabetic nephropathy and hypertensive nephropathy
In the last 20 years, in the general population, there has been an increase in the frequency of end-stage renal disease secondary to diabetic nephropathy and hypertensive nephroangiosclerosis, reaching 70% of all cases of end-stage renal disease diagnosed. The metabolic complications of ART (dyslipidemia, body fat changes, insulin resistance, DM) and the ageing of the infected population suggest that renal damage secondary to DM and HBP may be increasingly important in HIV-infected patients79. In some series of renal biopsies of HIV-infected patients, diabetic nephropathy has been reported in 6% of cases and hypertensive nephropathy with nephroangiosclerosis in 4% of cases80,81.
Bearing in mind that in HIV-infected patients a high prevalence of albuminuria (an established predictor of cardiovascular disease [CVD] and renal disease) has been reported and a close association between the amount of albuminuria and traditional CVR factors such as insulin resistance and HBP82 has been observed, a growing incidence of diabetic nephropathy and hypertensive nephropathy is to be expected in these patients over the coming years.
3.2.1.4. Thrombotic microangiopathy
The incidence of thrombotic microangiopathy (TMA), with renal and/or neurological involvement, is probably higher in HIV-infected patients than in the general population83-86. Various HIV proteins may directly damage endothelial cells, inducing apoptosis84, and in HIV-infected test animals TMA frequently develops, which makes us suspect that HIV may perform a key role in TMA-associated endothelial damage87. The clinical course is aggressive and the prognosis is sombre. Symptoms are similar to those observed in TMA cases in patients without HIV infection83-86.
Most patients are young and male and the progressive deterioration of renal function is accompanied by hematological findings that are typical of TMA: anemia with schistocytes in peripheral blood, thrombocytopenia, increased lactate dehydrogenase (LDH) and decreased haptoglobin. Although most cases have a clear and rapidly progressive deterioration of renal function, similar to the symptoms of hemolytic-uremic syndrome, in others there may be predominant neurological manifestations, as is the case in thrombotic thrombocytopenic purpura. Renal function deterioration, if there are no concurrent glomerular processes, may manifest as progressive oligoanuria with low proteinuria and mild urinary abnormalities. In other cases, by contrast, macroscopic hematuria and nephrotic-range proteinuria can be observed. The renal biopsy shows similar changes to those of idiopathic TMA. In most cases, there is irreversible renal failure and mortality is very high. Plasmapheresis, the administration of fresh plasma and more recently eculizumab, a specific inhibitor of the complement attack complex, are the treatments recommended in hemolytic-uremic syndrome88, but there is no information about their efficacy in HIV infection-associated TMA.
3.2.1.5. Malignant hypertension
Malignant hypertension is defined as very high blood pressure (BP) values with grade III or IV hypertensive retinopathy. It has been reported to be associated with various glomerular diseases in HIV-infected patients89,90, amongst them IgA-induced nephropathy, membranoproliferative GN, membranous nephropathy and focal glomerulosclerosis. The relationship between hypertensive emergency and TMA is well-known. TMA may be accompanied by hypertensive emergency and hypertensive emergency may trigger TMA. Strict monitoring of BP, with an early introduction and high doses of renin-angiotensin system blockers (ACE inhibitors or ARBs), allows an improvement in the ARF that accompanies most cases of hypertensive emergency91. However, the prognosis of hypertensive emergency associated with HIV infection is considerably worse.
3.2.2. Tubular and interstitial nephropathies
HIV-infected patients may have a wide variety of tubular and interstitial nephropathies. The main forms are described in the following paragraphs.
3.2.2.1. Acute tubular necrosis
In many cases of prerenal ARF secondary to intercurrent diseases, when the underlying cause is not quickly and adequately corrected, acute tubular necrosis may occur. Likewise, the intrinsic tubular nephrotoxicity of certain medications and radiographic iodinated contrasts may cause tubular necrosis in HIV-infected patients. Recovery may take days or weeks, in accordance with the degree of damage, although it is not always complete. Acute tubular necrosis should be considered when there is progressive ARF, normally (but not always) with diuresis maintained, in the context of a septic and/or hemodynamically unstable patient, or when iodinated contrast or any of the drugs potentially involved, such as NSAIDs, angiotensin receptor blockers, aminoglycosides, trimethoprim-sulfamethoxazole, pentamidine, amphotericin B, foscarnet, cidofovir or TDF have been administered.
3.2.2.2. Drug-induced tubular nephropathies
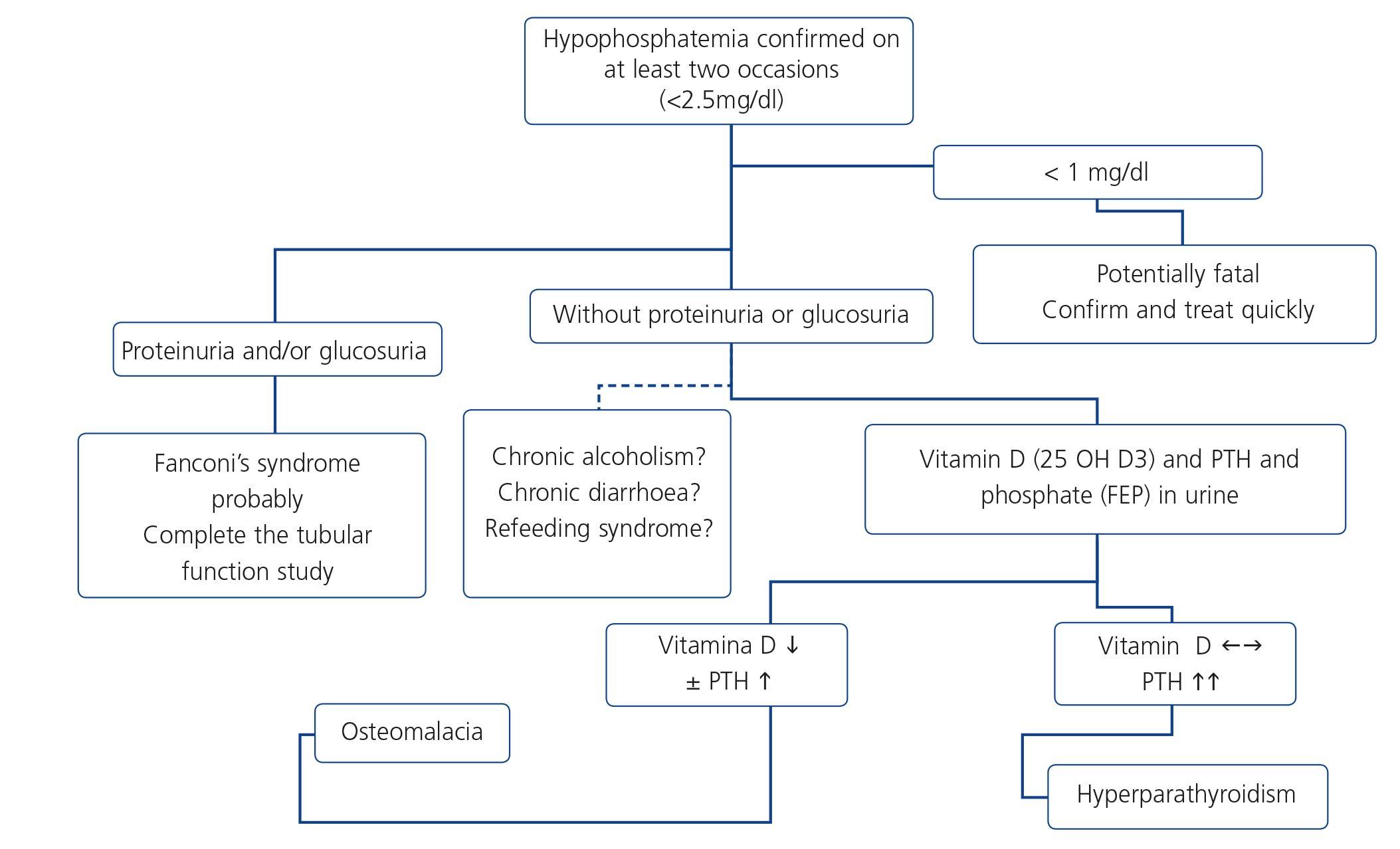
Fanconi’s syndrome is the best characterized clinical expression of the damage and dysfunction of the proximal tubular cells of the kidney. The most extreme form is characterized by a generalized deficiency in reabsorption in the proximal tubule, favouring urinary loss of phosphate, calcium, urate, amino acids, glucose and bicarbonate, amongst others. This may be expressed as disproportionate phosphaturia and uricosuria, with the development of hypophosphatemia and hypouricemia, aminoaciduria, glucosuria in spite of normoglycemia and type II renal tubular acidosis (metabolic acidosis with normal anion gap), as well as tubular proteinuria (normally less than 2g/day), hypokalemia and polyuria and polydipsia due to the inability to concentrate urine. When in addition to functional damage of the proximal tubular cell there is structural damage and apoptosis of the latter, there is tubular necrosis and renal failure, which if it persists over time due to the original damage being maintained, may become chronic. The normal situation, however, is that the symptoms of the syndrome occur in an incomplete manner, mainly as variable hypophosphatemia, normoglycemic glucosuria and proteinuria.
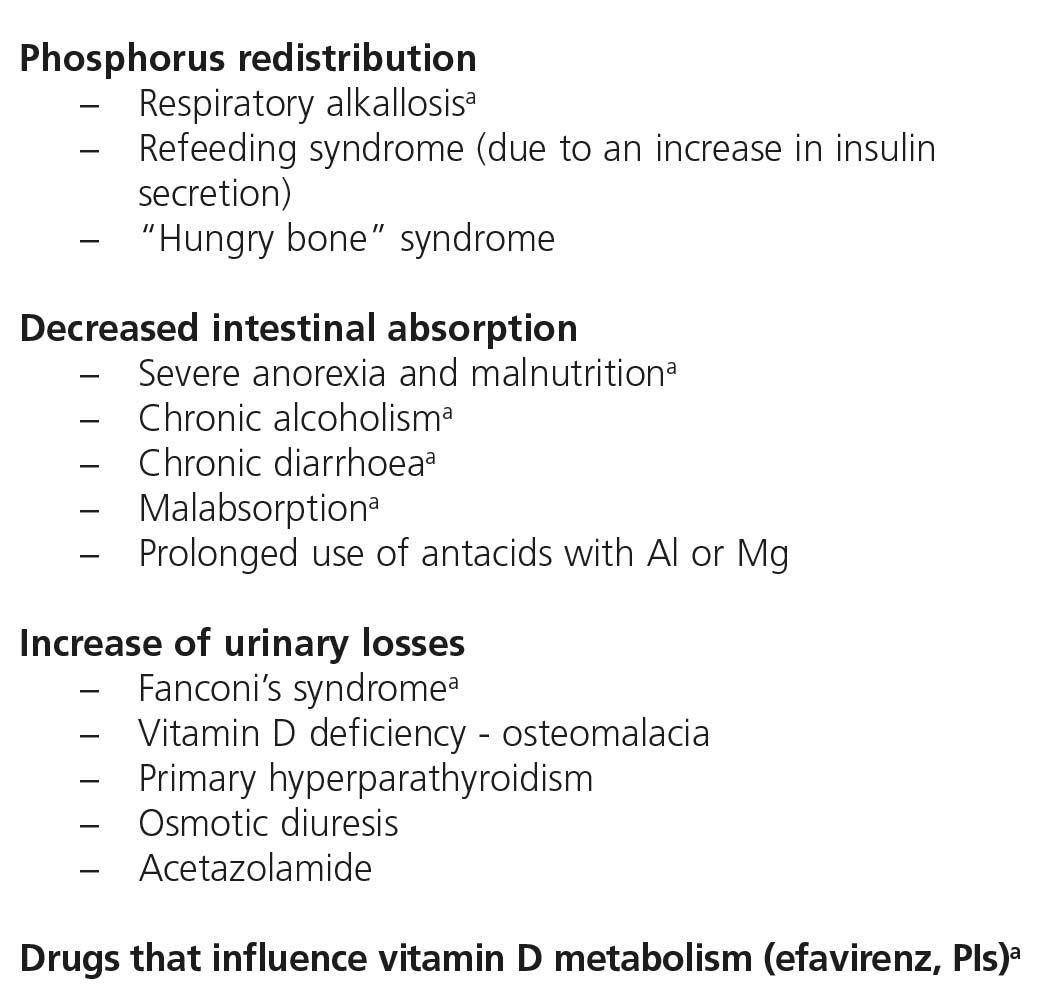
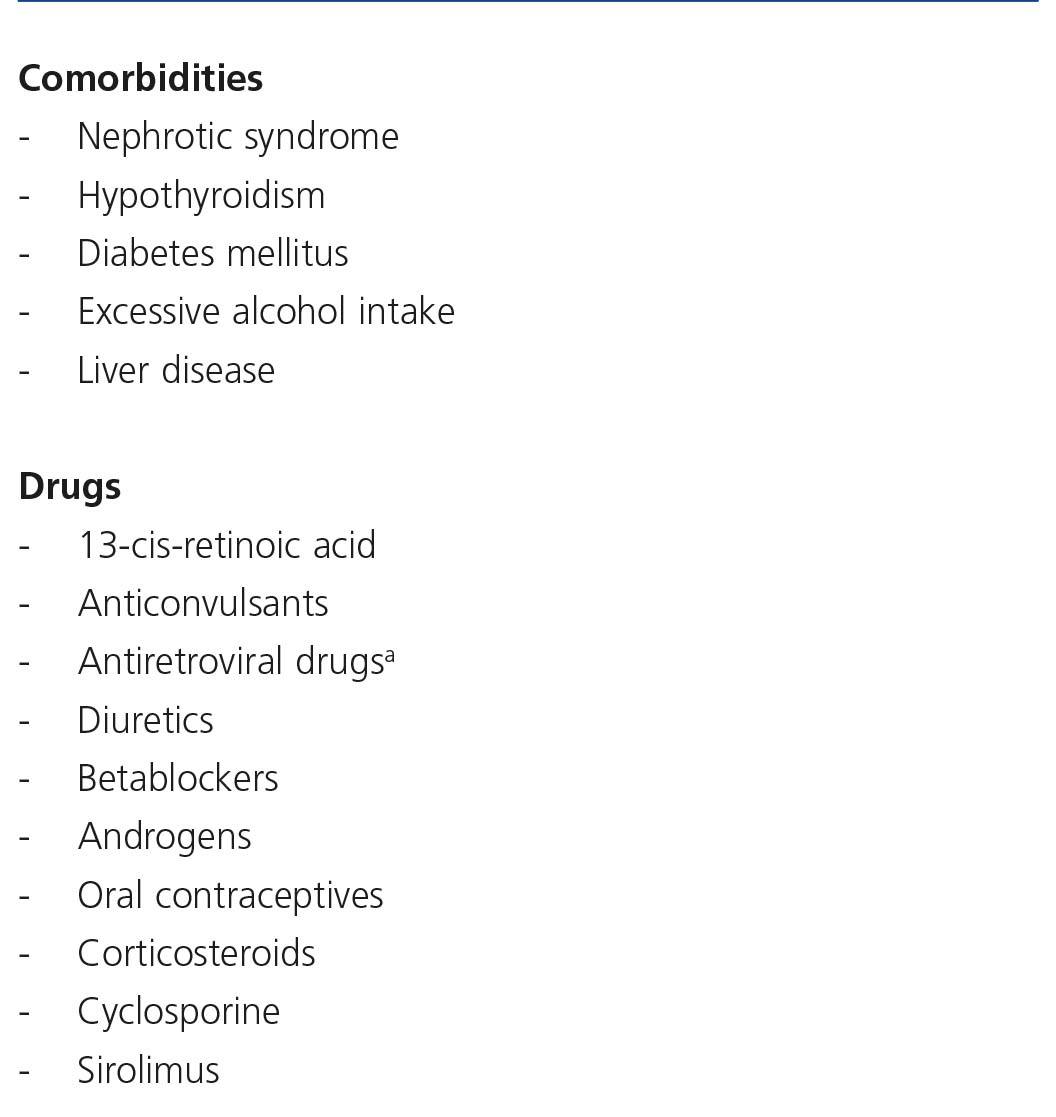
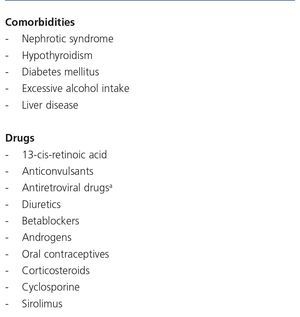
There are many causes of complete or partial Fanconi’s syndrome, with there being two main groups: congenital, presenting in infancy, and acquired, presenting predominantly in adulthood, related to paraproteinemias, tubulointerstitial diseases and drugs or toxic substances (Table 5). In HIV-infected patients, the most common cause of Fanconi’s syndrome is the use of drugs, mainly TDF92,93 and less commonly, other nucleoside analogues, such as didanosine (ddi) and stavudine (d4T)94-96. Other drugs well associated with Fanconi’s syndrome are adefovir, used for treating HBV infection, and cidofovir, used for treating citomegalovirus infections.
Various risk factors have been identified for the development of nephrotoxicity with the use of TDF, including previous CKD, joint administration with other nephrotoxic drugs, low body weight, older age and a low CD4 lymphocyte count97. In some studies, a history of opportunistic infections, the presence of “comorbidities”, HBP, NSAID administration98, chronic pain (a marker of NSAID use), the simultaneous use of ddi and the co-administration of boosted PIs99-103 have also been associated with a greater risk of nephrotoxicity with the use of TDF. Boosted PIs may decrease renal clearance of TDF and favour its accumulation in the tubular epithelial cells104. In a recent review of 164 cases of Fanconi’s syndrome associated with TDF, 84% were simultaneously receiving PIs, in most cases boosted with RTV92. Lastly, certain polymorphisms in genes that encode TDF transport proteins in proximal renal tubule cells, specifically in certain haplotypes of genes ABCC2 (MRP2) and ABCC4 (MRP4)105-107, have also been associated with a greater risk of nephrotoxicity with the use of this drug.
3.2.2.3. Immunoallergic interstitial nephritis
In addition to direct tubular damage, some drugs may cause ARF as a result of an immunoallergic reaction characterized by a diffuse interstitial infiltrate rich in eosinophils. The presence of peripheral eosinophilia, skin rash and fever must suggest this diagnosis in patients with ARF in which the cause is not clear, particularly in the presence of non-nephrotic proteinuria, leukocyturia and leukocyte casts, in the absence of urinary infection and when a new treatment has recently been introduced108. Renal biopsy is the only form of establishing the diagnosis, although in elderly patients or those with severe associated problems, it is common to opt for empirical treatment without renal biopsy108. The quick withdrawal of the drug responsible is the principal basis of the treatment. In addition, a short course of steroids (4 to 6 weeks) favours the complete recovery of renal function109. Antibiotics and NSAIDs are the drugs that most commonly cause immunoallergic nephritis, although any drug, including antiretroviral drugs, may trigger it110.
3.2.2.4. Crystal deposition-induced intrarenal obstructive nephropathy
This is caused by the mass deposition of crystals and their potential obstruction in the tubules after treatment with drugs with low solubility in urine, especially at high concentrations and specific pH values111. The drugs involved in HIV-infected patients may be sulfadiazine, IDV, ATV, foscarnet and acyclovir in high doses. Nephrolithiasis has also been reported with IDV and ATV due to an accumulation of the crystals excreted. The presence of crystalluria has been noted in patients treated with DRV111, but to date there have been no documented cases of obstructive nephropathy due to crystal deposition in patients treated with this PI. Adequate hydration is important in preventing and treating this complication, which is usually reversible, although the inflammatory reaction secondary to crystals themselves may cause varying degrees of persistent chronic tubulointerstitial damage.
3.2.2.5. Rhabdomyolysis
Acute muscle damage caused by drugs, ischemia secondary to compartment syndrome, sepsis, or water and electrolyte disorders is not uncommon in HIV-infected patients. Myoglobin released and filtered in the glomerulus produces characteristic ARF, with dark urine, a massive increase in muscle enzymes and renal tubular obstruction and necrosis due to myoglobin casts.
3.2.3. Non-specific chronic renal failure
In addition to glomerular and tubulointerstitial processes, whose clinical manifestations are generally very obvious, in recent years a high incidence of CKD has been reported in HIV-infected patients, characterized by GFR decreases of varying severity, not accompanied by significant proteinuria or sediment abnormalities that are suggestive of glomerular disease. The cause of CKD in these patients is probably multifactorial: in some patients, it may be the consequence of previous ARF episodes that have not completely resolved, and in others, the nephrotoxic effect of certain treatments maintained for years, including certain antiretroviral drugs. The clinical-laboratory profile of this form of CKD in HIV-infected patients is very similar to that of “silent” or “occult” CKD, whose incidence in patients of advanced age, otherwise normal, is high (up to 10%-33% in patients older than 70 years of age)112. In the general population, this “occult CKD epidemic” has been related to chronic disorders (HBP, DM) and to the renal changes attributable to old age. These factors may play a role in HIV-infected patients at an earlier age, due to mechanisms that are not yet well-known. It is interesting to note that in HIV-infected patient groups who have not received ART, this high CKD prevalence has also been reported, without HBP or DM providing satisfactory explanations113.
4. RENAL EVALUATION OF HIV-INFECTED PATIENTS
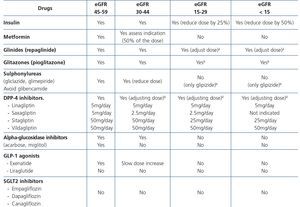
The prevention of renal damage in HIV-infected patients must include acting on modifiable CKD risk factors (Table 6), detecting occult CKD, identifying the CKD aetiology and acting on factors that influence both its development (Table 6) and its progression (Table 7), paying particular attention to drugs, both antiretroviral and others used to treat the complications associated with HIV infection. The objectives of regular evaluation of renal function and of the presence of renal damage markers are early detection of renal disease, its follow-up and the adjustment of doses of nephrotoxic drugs or those that are eliminated through the kidneys.
From an operational point of view, in these recommendations, the tests used to evaluate renal function were classified as a “basic renal study or screening”, which it is advised to carry out on all HIV-infected patients, and a “comprehensive renal study”, which must be carried out on selected patients. In the anamnesis, we should remember the importance of recording a personal or family history of nephropathies and high-risk factors for developing them (Tables 6 and 7), as well as recording BP and body weight.
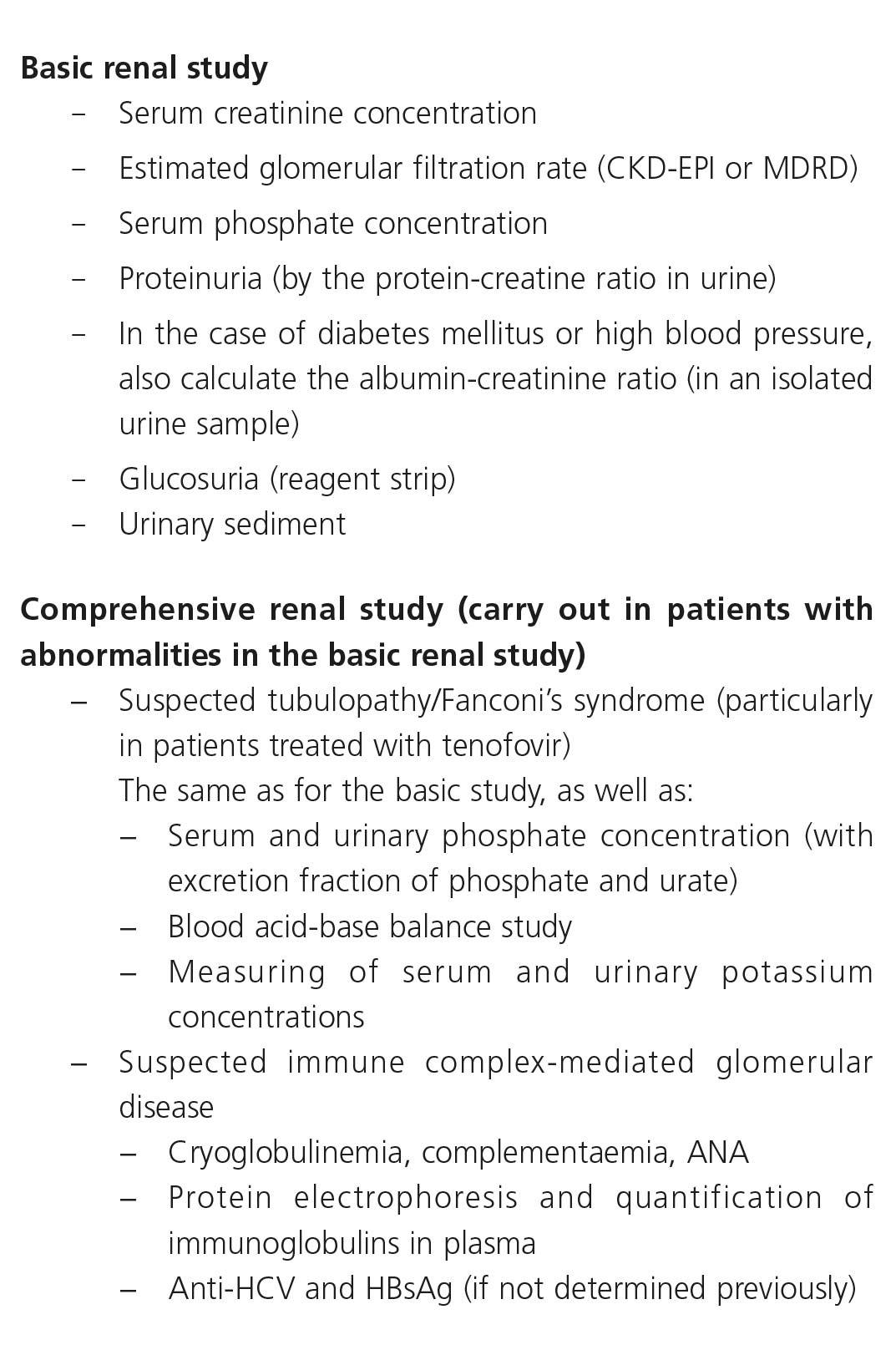
The basic renal study will include measuring serum creatinine concentration and estimating GFR, measuring protein/creatinine ratio (PCR) and ACR (in the case of DM or HBP), both in the first-morning-void urine sample, as well as urinary sediment and carrying out a basic evaluation of tubular function (serum phosphate concentration, proteinuria as mentioned above and glucosuria by test strip) (Table 8).
With regard to the comprehensive renal study, when an abnormality in the basic study has been detected, whether it be an abnormal GFR, a decrease in serum phosphate or the presence of proteinuria, glucosuria or hematuria, it is necessary to attempt to identify the cause and determine the associated factors and prognosis. In these cases, a comprehensive study of markers in blood and urine (Table 8) may be required and possibly imaging tests and renal biopsy.
Consequently, in the case of potential tubular involvement secondary to antiretroviral toxicity, the serum concentrations of urate, potassium and bicarbonate (or the acid-base balance) will be determined, as well as the urine concentrations of phosphate, urate and potassium, and the fractional excretion and tubular reabsorption of phosphate (TRP) and urate will be calculated. In the case of suspected glomerular disease, specific studies will be carried out in accordance with whether the glomerulopathy is suspected to be primary or secondary: immune complex-induced glomerular disease and lupus, diabetic or hypertensive nephropathy, etc.
Below we describe in detail each laboratory test that is to be performed in the evaluation of the renal function of HIV-infected patients.
4.1. Measurement of serum creatinine concentration and estimation of the glomerular filtration rate using the CKD-EPI equation
Serum creatinine concentration is the most used biomaker for assessing renal function. However, it has significant interindividual variability mainly due to differences in age, sex and muscle mass, which limits the usefulness of population reference values in the early detection of renal function abnormalities.
The equations for estimating the GFR that include, in addition to serum creatinine concentration, other variables such as age, sex and racial group is currently considered to be the best form of evaluating renal function. In HIV-infected patients, as in the rest of the population, GFR estimation using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation114 is advised and it has demonstrated its superiority over other GFR estimation equations based on serum creatinine concentration (MDRD, Modification of Diet in Renal Disease), cystatin C or in the combination of both115-117. The CKD-EPI equation underestimates the true GFR value less than the MDRD equation, which allows the eGFR values that can be reported by this equation118 to be extended to 90ml/min/1.73m2. This may be of great interest in HIV-infected patients, since in some cases, a dose adjustment may be required in eGFR segments between 60ml/min/1.73m2 and 90ml/min/1.73m2, a situation that was not possible with MDRD because this equation provided results with greater dispersion, especially with an eGFR >60ml/min/1.73m2, and as such, these values were only reported as “>60ml/min/1.73m2”, without an absolute value being specified.
In recent years there has been a standardization of most creatinine measuring procedures with the objective of decreasing their variability and the impact of the latter on GFR estimation119. This standardization has consequences regarding the type of equation that should be used120. At present, most Spanish clinical laboratories report the GFR value using the MDRD-IDMS (isotope dilution mass spectrometry) or MDRD equations, in accordance with whether the method for measuring creatinine is standardized or not121.
At present, the CKD-EPI equation, for standardized creatinine methods, is replacing MDRD-IDMS in laboratory reports. During this transition process, both equations may be used (Table 9). The Cockcroft-Gault (CG) equation122, classically used to adjust drug doses, cannot be re-formulated for the current methods of measuring creatinine, and as such, it should not be used. The CKD-EPI or MDRD-IDMS equations may be used for this purpose, since they are based on standardized creatinine measuring procedures, they relate better to the measured GFR than CG for GFR values <60ml/min/1.73m2, which are the values most liable to have dose adjustment, and are available in most clinical laboratory reports, unlike CG123-125.
Furthermore, for any GFR value, the MDRD or CKD-EPI equations are more accurate than the CG equation21,30,121. Another problem is the lack of studies that have used eGFR for drug adjustment, since to date, most express adjustments according to CrCl estimated by the GC formula. A study has recently shown the validity of MDRD for drug adjustment, even demonstrating it to be superior to CG125. Some ART dose adjustment guidelines126 already use the MDRD equation, indicating that the CG equation can be used as an alternative. In the near future, the guidelines for drug adjustment will probably use the eGFR in accordance with the recommendations of the scientific societies that use MDRD or CKD-EPI.
Measurement of serum cystatin C concentration has been proposed as a useful marker for assessing renal function, since its concentration is independent of muscle mass, it is filtered by the glomerulus and is completely reabsorbed by the renal tubule127. However, some studies have shown increases in serum cystatin C in HIV-infected patients associated with high concentrations of C-reactive protein (an inflammation marker that can be high in HIV-infected patients), a high viral load and a low CD4+ lymphocyte count128. The few studies carried out in HIV-infected patients, the heterogeneity in the results obtained (in part explained by the different methods used to measure the GFR, creatinine and cystatin C)116,117,129-132 and its high cost mean that cystatin C cannot currently be recommended as a marker of renal function in the screening and follow-up of HIV-infected patients.
The measurement of CrCl (using 24-hour urine) does not provide any advantage over GFR estimation using an equation, it is subject to greater variability, and 24-hour urine collection is inconvenient for patients. Its use should be reserved for situations in which a GFR estimation equation is not suitable, such as in individuals who follow special diets, those who have extreme body weight (body mass index <19kg/m2 or >35kg/m2), those with significant muscle mass abnormalities or patients with severe liver disease133.
In HIV-infected patients, serum creatinine concentration, and by extension, GFR value estimation through the equations that include it, may be affected by factors that are not due to actual changes in the GFR. Therefore, patients with significant decreases in muscle mass, severe malnutrition or advanced liver disease secondary to HCV or HBV infection may have decreases in serum creatinine concentration, with the resulting overestimation of the eGFR value. Likewise, certain drugs can cause an increase in creatinine concentration by inhibiting its active tubular secretion. This effect causes a false decrease in the eGFR using the MDRD and CKD-EPI equations, without there being a true decrease in the actual GFR. The main drugs involved that may be prescribed in HIV-infected patients are trimethoprim (normally coformulated with sulfamethoxazole), cimetidine (an antacid seldom prescribed at present) and various new generation antiretroviral drugs, such as RPV and DTG or boosters of these drugs, such as COBI134,135. Various studies have observed that their use is accompanied by a slight increase in serum creatinine concentration, without a change in the GFR measured by isotopic methods. This characteristic may be a matter of concern, particularly if they are administered in combination with potentially nephrotoxic drugs such as TDF. Neither the estimation of the GFR using the equations that include serum creatinine concentration or the calculation of CrCl in 24-hour urine are useful in these circumstances. In these cases, the use of other more complex renal function markers (such as serum cystatin C concentration) or isotopic methods if an accurate measurement of the GFR is required, may be useful. These methods, as well as being expensive, are not accessible to all hospitals.
To distinguish between an increase in creatinine due to the blocking of its tubular secretion and an actual deterioration of the GFR, the following considerations must be borne in mind:
1. The potential effect of the abovementioned drugs on serum creatinine concentration.
2. The increase in serum creatinine concentration due to the blocking of its tubular secretion:
•It must be small, normally <30% of the initial creatinine concentration.
•It occurs at an early stage after the administration of the drug, commonly after a few days, and subsequently remains stable if the patient’s clinical and metabolic situation does not change. To confirm stability, it is recommended to repeat a creatinine concentration measurement within a maximum period of four weeks.
•After the drug is withdrawn, serum creatinine concentration returns to its baseline values.
•Serum urea concentration will remain stable, which is not the case with creatinine.
•It will not be accompanied by proteinuria, normoglycemic glucosuria or urinary sediment abnormalities.
Other drugs such as fibrates are associated with an increase in serum creatinine concentration due to mechanisms that are not well-known136,137.
4.2. Measurement of the protein/creatinine ratio and/or the albumin/creatinine ratio in the first urine of the morning
The persistently high concentration of protein or albumin in urine is a sign of renal damage and along with a decreased GFR, they are the most used criteria for diagnosis and classification in CKD stages. Small increases in protein concentration in urine usually precede an increase in serum creatinine concentration or a decrease in the GFR. Proteinuria may occur in up to 30% of HIV-infected patients76,138. It is a better marker of progression to end-stage renal disease than a decrease in the GFR139 and a CVR and mortality factor28. For the selection of the biological scale to be used in the evaluation of proteinuria, both the clinical context of the patient and methodological aspects related to albumin and protein measurement in urine must be considered. From a clinical point of view, HIV-infected patients may have:
• Glomerular proteinuria, secondary to various glomerulopathies or diabetic or hypertensive nephropathy, predominantly characterized by increased elimination of albumin in urine.
• Tubular proteinuria, secondary to tubulointerstitial nephropathies or toxicity associated with antiretroviral therapy, characterized by an increase in the urinary concentration of low-molecular-weight proteins such as α1-microglobulin, β2-microglobulin, retinol-binding protein (RBP), n-acetyl β-glucosaminidase (NAG) or neutrophil gelatinase-associated lipocalin (NGAL).
• Mixed proteinuria (glomerular and tubular) due to the coexistence of the processes described above.
From a methodological point of view, the following should be taken into account:
• The procedures for measuring protein in urine mostly recognize albumin and are less sensitive to other proteins such as globulins and low-molecular-weight proteins, which must be present in relatively high concentrations to be detected.
• The procedures for measuring albumin are more sensitive in laboratory tests and are more standardized than those of protein140,141.
• The use of the urine test strip for the screening of proteinuria in HIV-infected patients is a semi-quantitative screening method and is not advised because its laboratory test sensitivity is lower than that of quantitative methods, it is particularly sensitive to albumin and less sensitive to globulins and low-molecular-weight proteins, and there may be false negative results in diluted urine and false positive results in concentrated, alkaline (pH>7), hematuric urine or urine with coloured components. In a study carried out in HIV-infected patients, 21% of patients with significant proteinuria (>300mg/g) were not detected with the test strip but were detected with the PCR142. The urine test strip may be useful for assessing the presence of hematuria, leukocyturia and glucosuria.
• The specimen of choice is random urine, preferably the first urine of the morning, since it has shown a good correlation and concordance with the values obtained in 24-hour urine, with the exception of nephrotic range proteinuria (>3g/day), in which the recommended specimen is 24-hour urine143.
• The expression of the results as ACR or PCR ratios instead of as concentrations avoids the errors resulting from a higher or lower dilution of the urine sample in relation to diuresis.
• The methods that specifically measure low-molecular-weight proteins are not available in most clinical laboratories.
The determination of albuminuria and proteinuria may be influenced by certain clinical conditions that modify their values. Some situations increase the albuminuria value detected: intense physical exercise, active infection, fever, hyperglycemic decompenzation or heart failure.
It must be borne in mind that albuminuria excretion by ACR or PCR may be overestimated in patients with decreased muscle mass. By contrast, in very muscular individuals or in Black individuals (African Americans), it may be underestimated32. In these extreme cases (weight and muscle mass), the determination of proteinuria/albuminuria in 24-hour urine may help provide a better interpretation of the renal evaluation.
Recently, the use of the albumin-to-protein ratio (APR) has been reported in an isolated urine sample, which may help to distinguish proteinuria of glomerular origin from that of tubular origin. An APR in urine >0.4 is suggestive of glomerular proteinuria (GN, HBP and DM), and an APR <0.4 is suggestive of tubular proteinuria, since most proteinuria is different from albumin, which will be tubular proteins144. These data were obtained from a study in patients with different renal diseases in whom the aetiological diagnosis was performed by renal biopsy. In these patients, total proteins in urine, albuminuria and tubular proteins (NAG and b-2 microglobulin) were determined. The ratio cut-off point of 0.4 had a sensitivity of 88% and a specificity of 99%.
These data were confirmed in a study in HIV-infected patients for distinguishing between tubular and glomerular proteinuria145.
4.3. The urinary sediment study
The presence of renal tubular cells, dysmorphic erythrocytes, erythrocyte casts and waxy casts is pathognomonic of renal damage and may indicate the diagnosis of certain pathologies.
4.4. Evaluation of tubular function
Tubular dysfunction in HIV-infected patients occurs mainly due to TDF-induced toxicity. If for various reasons (non-adjustment of the dose to the eGFR, the presence of renal toxicity risk factors, other drugs that boost nephrotoxicity or the existence of certain genotypes that influence TDF metabolization and favour tubular toxicity) this toxicity arises, there is an alteration of the physiological functions of the proximal tubule: reabsorption of proteins, phosphorus, glucose, uric acid, amino acids and bicarbonate and Fanconi’s syndrome will occur, which may be complete or (in most cases) incomplete. This syndrome may also result from causes other than TDF-induced nephrotoxicity (Table 5).
Consequently, the tubular dysfunction study will include, in addition to proteinuria, certain substances such as phosphate, uric acid and glucose, whose reabsorption is predominantly proximal.
Low-molecular-weight proteins are freely filtered in the glomerulus and consequently reabsorbed in the proximal tubule in patients without renal tubular pathology, with a small amount of proteins appearing in urine. When there is an abnormality in tubular reabsorption, the excretion of these proteins in urine increases. As there is no distal reabsorption of proteins, the urine measurement of low-molecular-weight proteins has been widely accepted as a marker of proximal tubular damage. These low-molecular-weight proteins constitute tubular proteinuria, which are proteins other than albumin (normally the main protein in urine). Therefore, the APR in urine is useful for differentiating between tubular and glomerular proteinuria144,145.
Proximal renal tubular dysfunction is a relatively common and generally reversible complication of long-term treatment with TDF in combination with PIs. Fanconi’s syndrome is the main clinical profile and it manifests in 0.3%-2% of patients. The isolated presence of tubular function abnormalities such as hypophosphatemia, normoglycemic glucosuria, proximal renal tubular acidosis or a decrease in the TRP are much more common (4%-50%, according to the series), but both their long-term and short-term clinical significance is uncertain146,147. The presence of high urinary concentrations of low-molecular-weight proteins such as β2-microglobulin and the RBP has been observed in up to 70% of patients as a result of mitochondrial dysfunction associated with treatment with TDF. Furthermore, tubular function disorders, even major forms, may go unnoticed until they lead to a decrease in the GFR. The clinical practice guidelines published are not homogeneous in terms of the recommendations on which diagnostic tests best identify the presence of tubular damage in these patients8,24,28,126.
In general, the existing recommendations suggest that the progressive decrease in the eGFR, an increase in the PCR value, the presence of hypophosphatemia or abnormalities detected in the urine and normoglycemic glucosuria test strip must make us suspect tubular damage and recommend that more specific tests be performed, which may include: measuring serum and urine phosphate and urate concentrations and calculating the respective fractioned excretions of serum bicarbonate, measured or calculated from the acid-base balance, and serum and urinary potassium (Table 8).
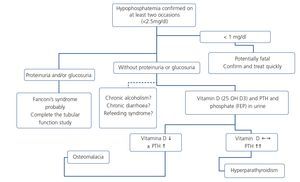
Hypophosphatemia may be due to many different causes of proximal tubulopathy (see section 7.5). In these cases, carrying out fractional excretion of phosphate (FEP) or of uric acid may assist in the diagnosis. A normal FEP (<20%) both of phosphate and of uric acid may indicate that the tubule is not damaged and not affected by TDF-induced toxicity. By contrast, a high FEP (>20%) is not so specific because it may be influenced by primary or secondary hyperparathyroidism, levels of 25(OH) vitamin D or other causes, which lower the specificity of the test.
To calculate FEP, calculators found on webpages (http://senefro.org/modules.php?name=nefrocalc) can be used. However, in many of them, FEP is not available but TRP is. It can be calculated directly, since FEP = 1 – TRP (Table 9).
Determining specific tubular function proteins such as the urinary concentration of RBP or β2-microglobulin may be of interest, although aspects such as their limited availability in clinical laboratories, the lack of standardization of these methods and sources of variation must be taken into account.
• Recommendations on renal evaluation in HIV-infected individuals
The evaluation of renal involvement in HIV-infected individuals will include:
1. Measurement of serum creatinine concentration and estimation of the GFR, preferably using the CKD-EPI equation or, failing this, the MDRD equation. (Quality of the evidence: High. Recommendation grade: Strong).
2. Determining PCR in urine, preferably first-morning-void urine sample, or failing this, a random urine sample is acceptable. In patients with DM and/or HBP, the ACR will also be determined. (Recommendation based on consensus).
3. Basic assessment of tubular dysfunction by previous measurements (point 1 and 2), serum phosphate concentration and glucosuria detection by test strip, preferably in the first urine of the morning. (Recommendation based on consensus).
4. When abnormalities have been confirmed in any of the previous tests (basic study) due to the suspicion of antiretroviral-induced nephrotoxicity, more specific studies or a comprehensive study is advised, such as measuring the serum and urinary concentration of phosphate and urate, accompanied by the calculation of the respective fractional excretions; the acid-base balance in blood study and the measurement of serum and urinary potassium concentration. If glomerular disease is suspected, specific studies will be carried out in accordance with whether a primary or secondary glomerulopathy is suspected. Furthermore, imaging tests or consultation of the Nephrology department will be considered in accordance with the referral criteria, which are described in this document. (Recommendation based on consensus).
5. The urine test strip may be useful for detecting the presence of urinary infection (esterase and nitrites), tubular abnormalities (non-hyperglycemic glucosuria) or urinary sediment abnormalities (hematuria), and must not be used for assessing proteinuria. (Recommendation based on consensus).
6. The specimen of choice is random urine, preferably the first urine of the morning, since it has shown a good correlation and concordance with the values obtained in 24-hour urine, with the exception of nephrotic range proteinuria (>3g/day), in which the recommended specimen is 24-hour urine. (Recommendation based on consensus).
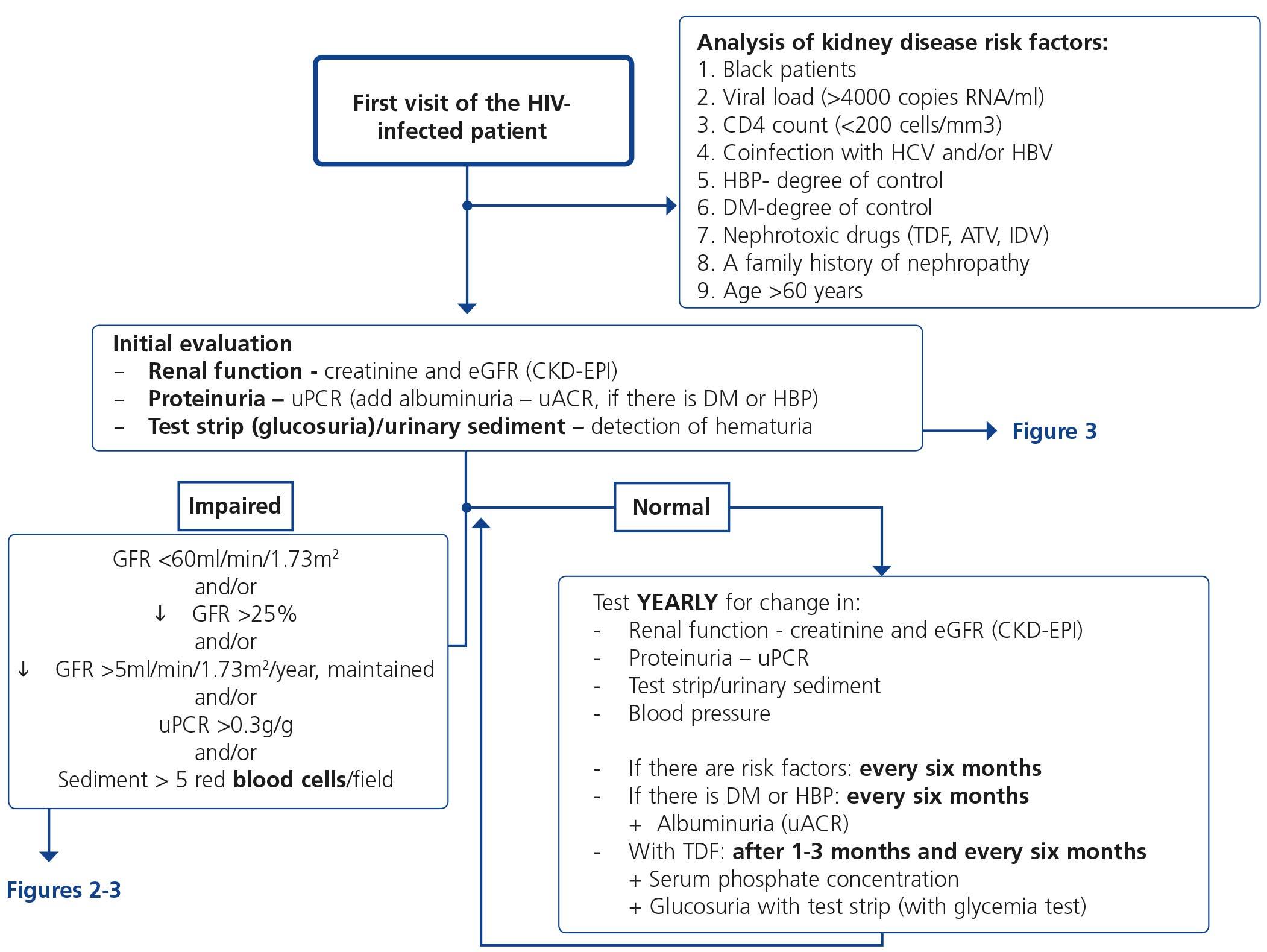
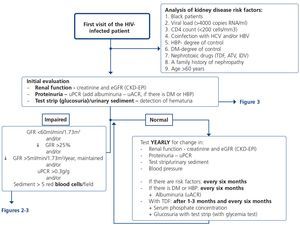
5. HOW REGULARLY SHOULD THE BASIC RENAL STUDY BE PERFORMED? (Table 10 and Figure 1)
The basic renal study should be carried out in all HIV-infected patients without exception, since there are many and frequent factors that favour the development of nephropathy. This is a low-cost study and does not involve additional hospital visits for patients, which supports the recommendation to carry it out in a general manner and on a regular basis.
This basic study is to be carried out in the first visit after diagnosis of the HIV infection, immediately before beginning ART and during the subsequent follow-up. The regularity with which it should be repeated during follow-up depends on the existence or not of risk factors for developing nephropathy (Tables 6 and 7). Given the simplicity and low cost, this group recommends that it be carried out in all scheduled check-ups of patients who receive ART in order that it may be included in the protocol or laboratory profile routinely performed in HIV-infected patients, and particularly in those who take TDF. In individuals with risk factors for renal disease (Figure 1, Tables 6 and 7), follow-up should be carried out every six months, and in those who have DM or HBP, albuminuria test (ACR) must be added to the yearly evaluation. In individuals treated with TDF, due to its potentially toxic effect on the renal tubule, renal function and proteinuria tests must be carried out one month after the drug is introduced, and subsequently every six months, in addition to the determination of serum phosphate and glucose using a urine test strip, preferably in the first-morning-void urine sample, although it can also be valid in a random urine sample (with a glycemia test). It has also been suggested that the first test after introducing ART be carried out after 2-3 months instead of after one month126. When abnormalities have been confirmed in any of the previous tests, it is advised to carry out a comprehensive study (see section 4). In the event of deterioration of renal function, proteinuria and/or hematuria, the patient should be assessed as set out in the following paragraphs.
Obviously, the regularity of these tests may be increased depending on the clinical opinion of the doctor who carries out the patient follow-up, particularly in those who receive treatment with TDF.
• Recommendations on the regularity of renal function tests:
1. In all HIV-infected patients, the basic renal study should be carried out to detect renal disease at the start of diagnosis of the HIV infection, and systematically in its subsequent follow-up. (Quality of the evidence: High. Recommendation grade: Strong).
2. This study should be carried out in patients who do not receive ART: when HIV-infection is diagnosed; once per year if there are no risk factors for the development of nephropathy; every six months when one or more of these factors are present; and before beginning ART. (Quality of the evidence: Low. Recommendation based on consensus).
3. In individuals treated with TDF, the frequency of tests must be increased. In patients without CKD risk factors, it is recommended to perform tests coinciding with those carried out for the efficacy of ART (1-3 months and subsequently every 6 months). In patients with CKD or CKD risk factors, it is recommended to advance the test to the month in which the drug is introduced. Each test will include the determination of serum phosphate and glucose and the urine test strip, preferably in the first urine of the morning (non-hyperglycemic glucosuria). (Quality of the evidence: Low. Recommendation based on consensus).
4. In patients treated with the coformulation of tenofovir, emtricitabine, cobicistat and elvitegravir (TDF/FTC/COBI/EVG, Stribild®), the European Medicines Agency (EMA) recommends monthly tests during the first year and subsequently every 3 months. (Quality of the evidence: High. Recommendation grade: Strong).
5. In the absence of abnormalities in the basic study, an annual follow-up is recommended that includes: measurement of serum creatinine concentration, estimation of the GFR (preferably using the CKD-EPI equation), determination of the PCR in the first urine of the morning and the urinary sediment study. (Quality of the evidence: Low. Recommendation based on consensus).
6. INDICATIONS FOR RENAL BIOPSY AND REFERRAL TO NEPHROLOGY
• Recommendations for performing a renal biopsy
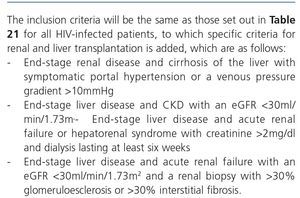
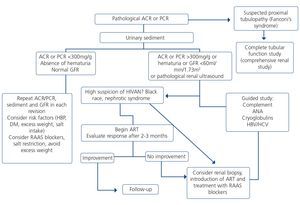
The indication of a renal biopsy will always be individualized in accordance with the balance between the risk of the biopsy and the benefits that it may bring. There is no evidence that HIV-infected patients have more complications related to renal biopsy than non-infected patients148. Biopsies are of interest not only in terms of diagnosis but can also show histological characteristics suggestive of progression or chronicity, particularly in patients who receive ART or other potentially nephrotoxic drugs, driving changes in drug therapy.148,149. However, and since early ART provides the best results for HIVAN, for patients in whom there is a very high suspicion of HIVAN when other causes of renal dysfunction/proteinuria have been ruled out, the doctor may opt not to carry out a renal biopsy. If there is no response (decrease in proteinuria or improvement of renal failure) in a reasonable period (6-8 weeks), a biopsy may then be considered.
Generally, renal biopsy indications are the same as for patients without HIV infection:
1. Nephrotic syndrome: PCR >3-3.5g/g (or its equivalent, proteinuria >3.5g/24 hours) accompanied by hypoalbuminemia <3g/dl.
2. Nephritic syndrome: edema, HBP, hematuria (macroscopic in many cases), oliguria, variable deterioration of renal function.
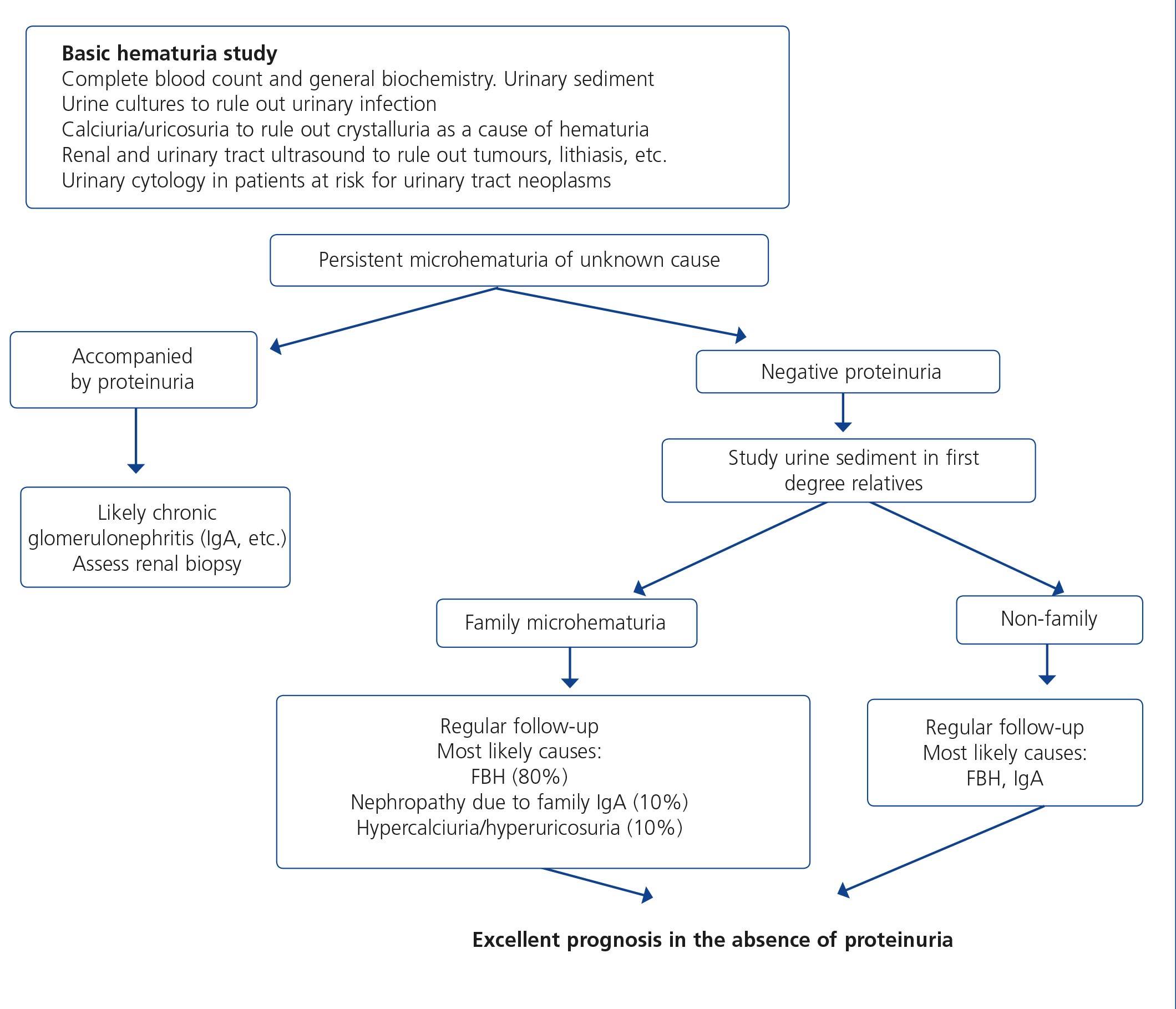
3. Persistent urinary abnormalities: significant asymptomatic proteinuria (PCR >1g/g), particularly when it does not decrease to <1g/g with ACE inhibitors, ARBs or other antiproteinuric measures (explained below) or when it is accompanied by urinary sediment abnormalities or deterioration of renal function. In cases with a PCR <1g/g (equivalent to 1g/24 hours), in isolation or accompanied by microhematuria, a conservative approach is recommended, with the use of renin-angiotensin system blockers (ACE inhibitors, ARBs) and scheduled follow-up. Renal biopsy will be considered when it is accompanied by major and persistent abnormalities of the sediment (persistent microhematuria and/or with intercurrent outbreaks of macroscopic hematuria). In this regard, the laboratory tests may express the results of red blood cells in urine in accordance with the number of red blood cells per field (40x field) or in the number of red blood cells per volume, usually in microlitres (µl). Expression by volume is that which is generally given by the analysers that calculate sediment automatically, which count particles per volume unit.
Although there is no standardization in the possible conversion factor that relates red blood cells/field and red blood cells/µl, very generally we can say that 1-2 red blood cells/40x field is equivalent to 4-8 red blood cells/μL In cases of persistent isolated microhematuria, renal biopsy is not advised, except in cases with microhematuria accompanied by signs and/or symptoms suggestive of systemic diseases. In diabetic patients with slowly progressive proteinuria and other data suggestive of diabetic microangiopathy, biopsy is not considered necessary, except if it is associated with data that suggest an aetiology other than diabetic nephropathy.
4. ARF: renal biopsy is indicated in the following cases:
• Presence of proteinuria, macroscopic hematuria or abnormalities in the sediment suggestive of ARF of glomerular origin.
• Clinical suspicion of immunoallergic interstitial nephritis (chronological relationship with drugs, skin rash, eosinophilia, etc.).
• ARF of an unknown cause.
5. TMA and malignant hypertension: renal biopsy is indicated if there is acute deterioration of renal function, proteinuria/wide-ranging hematuria, and if the general situation allows it.
Before biopsy, the renal morphology must be assessed with ultrasound (biopsy would be contraindicated in most patients with unilateral renal agenesis, renal malformations, small kidneys). Any treatment with anticoagulants and antiplatelets should be discontinued ahead of time and a prior complete coagulation study should be carried out. In risk patients, such as in those with renal failure, it may be necessary to take measures to prevent bleeding (for example, intravenous desmopressin)150.
• Recommendations for indicating a renal biopsy:
1. The indication of renal biopsy must be individualized, considering the risks and benefits. (Quality of the evidence: Low. Recommendation based on consensus).
2. Renal biopsy indications in HIV-infected patients are the same as for non-infected patients. (Quality of the evidence: Low. Recommendation based on consensus).
Indications for renal biopsy are the following:
• Nephrotic syndrome (PCR >3g/g).
• Nephritic syndrome.
• Persistent urinary abnormalities (PCR >1g/g that does not respond to treatment with renin-angiotensin system blockers or if there is persistent microhematuria >25-50 red blood cells/field or outbreaks of hematuria).
• ARF suspected to be of glomerular, immunoallergic or unknown origin.
• TMA and hypertensive emergency.
6.1. Criteria for referral to Nephrology
Referral to a Nephrologist must be considered as a collaboration for the adequate interpretation and approach to renal problems, particularly when they are complex or require diagnostic or therapeutic approaches. Its objectives are the adequate diagnosis of renal problems, delaying progression of end-stage renal disease and reducing morbidity and mortality. Referral to a Nephrologist will be carried out bearing in mind the state of CKD, its speed of progression, the degree of proteinuria, associated comorbidities and the patient’s functional situation.
From a practical point of view, three fundamental aspects will be considered for their referral: eGFR, proteinuria and other reasons.
1. In accordance with proteinuria (PCR >0.5g/g or ACR >300mg/g): patients with moderate asymptomatic proteinuria (PCR >0.5g/g or its equivalent, greater than 0.5g/24 hours), significant asymptomatic proteinuria (PCR 1-3g/g or its equivalent 1 to 3-3.5g/24 hours) and nephrotic proteinuria (PCR >3g/g or its equivalent >3-3.5g/24 hours), as well as those with an ACR >300mg/g (or its equivalent, albuminuria greater than 300mg/24 hours) should be referred to Nephrology department.
2. In accordance with the eGFR: patients with an eGFR <45ml/min/1.73m2 will be referred.
3. Other reasons for referral:
•Non-urological hematuria (>25-50 red blood cells per field), particularly if it is associated with proteinuria.
•Acute renal function deterioration (decrease in the eGFR >25%) in two consecutive tests, once external factors have been ruled out (diarrhoea, vomiting, depletion due to diuretics in treatment with ACE inhibitors, ARBs, direct renin inhibitors or NSAIDs).
•Any acute renal deterioration with suspected GN or acute interstitial nephritis.
•Patients with progression of renal function deterioration (>5ml/min/1.73m2/year) without any explicable cause.
•CKD and HBP that is refractory to treatment (>140/90mmHg) with three drugs at full dose, one of them a diuretic.
•Abnormality in serum potassium concentration (>5.5mEq/l or <3.5mEq/l when the patient is not receiving diuretics).
•Anemia of renal origin: hemoglobin (Hb) <10.5g/dl with CKD despite correcting iron deficiency (transferrin saturation index [TSI] >20% and ferritin >ng/ml), once other causes of anemia have been ruled out.
• Recommendations for referring patients to Nephrology:
It is recommended to refer to a Nephrologist those patients presenting of the following abnormalities:
PCR >0.5g/g (>50mg/mmol), ACR >300mg/g (0.3g/g or 30mg/mmol).
GFR <45ml/min/1.73m2.
Non-urological hematuria (>25-30 red blood cells per field).
Acute renal function deterioration or progressive deterioration of uncertain aetiology.
CKD and HBP refractory to treatment.
Abnormal potassium values (>5.5mEq/l or <3.5mEq/l) when the patient is not receiving diuretics.
Anemia of renal origin.
(Quality of the evidence: Low. Recommendation based on consensus).
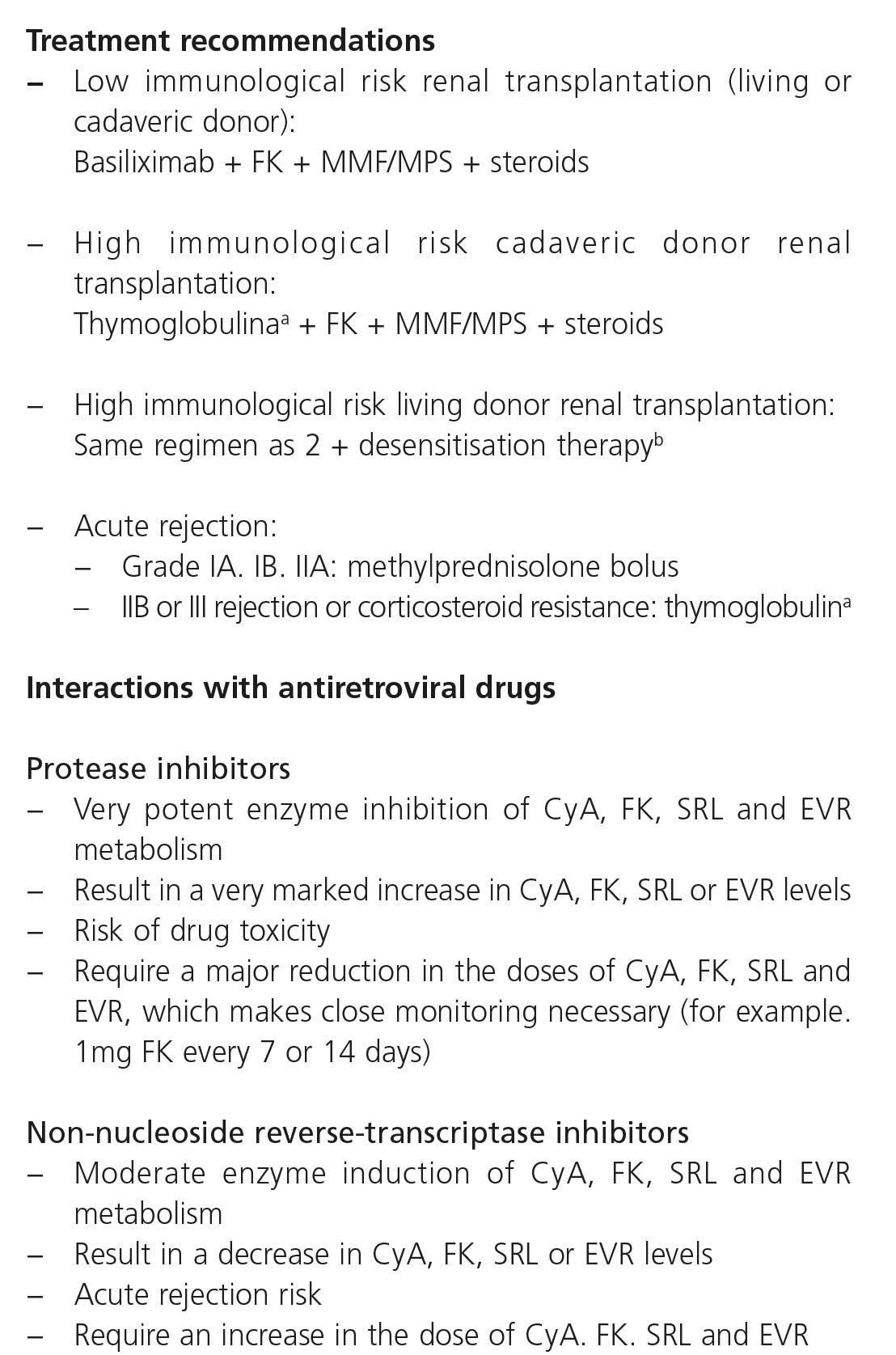
7. MANAGEMENT OF CHRONIC KIDNEY DISEASE PATIENTS
7.1. Diagnostic approach to suspected renal involvement in HIV-infected patients (renal impairment, hematuria, proteinuria)
7.1.1. Definition of renal function deterioration
Deterioration of renal function may occur in its acute form (ARF) or gradually, in which case we speak in terms of progression. ARF diagnostic criteria are clearly established (see section 3.1.1); however, there is major controversy over CKD progression criteria.
To evaluate progression, both GFR and proteinuria (or albuminuria) values must be considered, since both scales will be related to the speed of progression to more advanced CKD stages.
The following are considered to be progression criteria:
• The change to a more severe CKD stage accompanied by a decrease in the eGFR of >25 % with respect to the baseline value.
• A sustained decrease in the GFR >5ml/min/1.73m2/year32.
It should be borne in mind that small changes in the GFR are common, they do not necessarily indicate CKD progression and they are related to both biological variability and variability of the procedures for measuring serum creatinine concentration. A decrease in the GFR (<25%), which occurs as a result of water and salt depletion or the administering of renoprotective drugs that affect glomerular hemodynamics by blocking the renin-angiotensin-aldosterone system, should not be considered to be progression. A change to a higher albuminuria category or a more than 50% increase in the ACR with respect to the baseline value may be indicators of progression151.
7.1.2. Approach to renal function deterioration
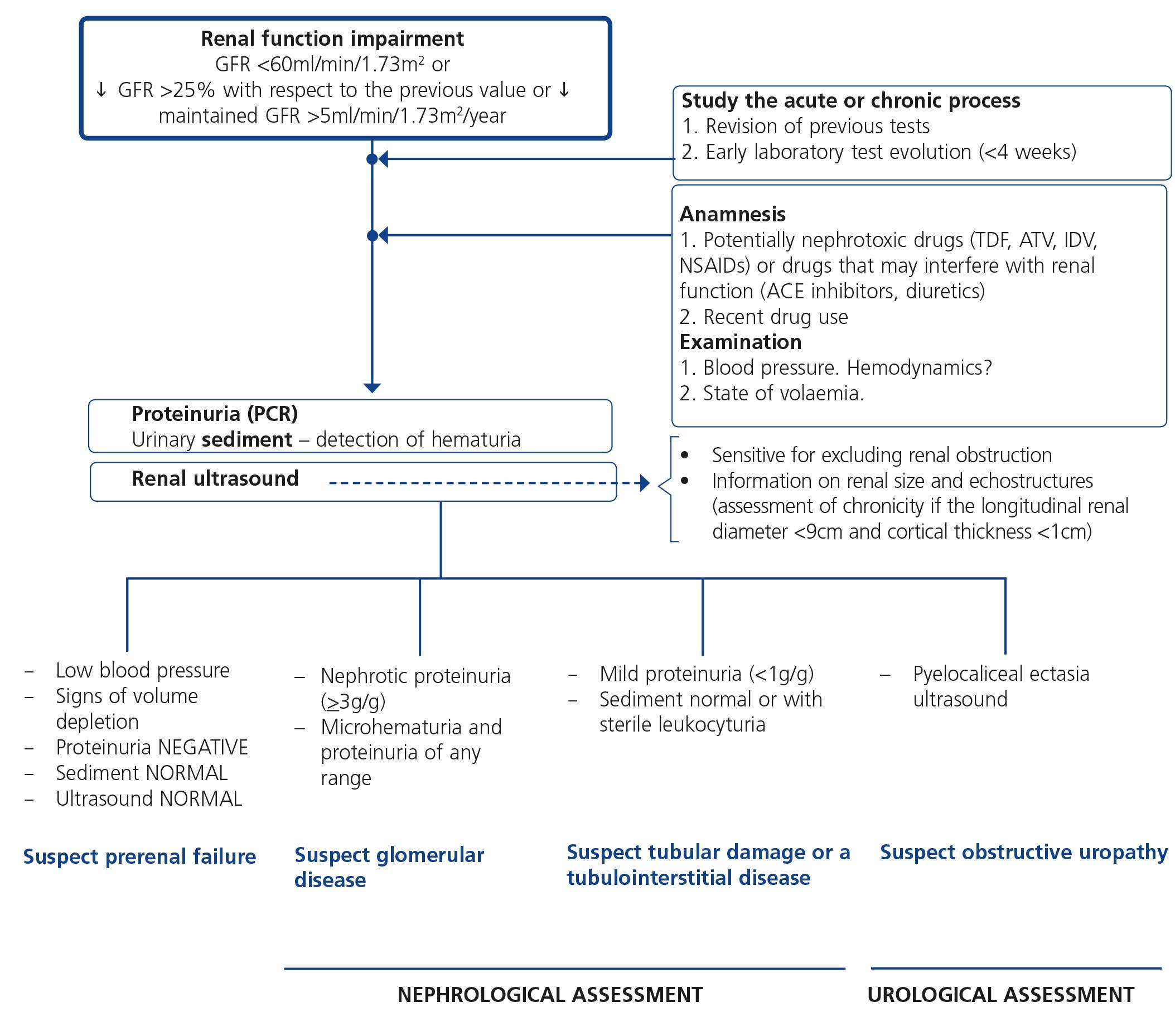
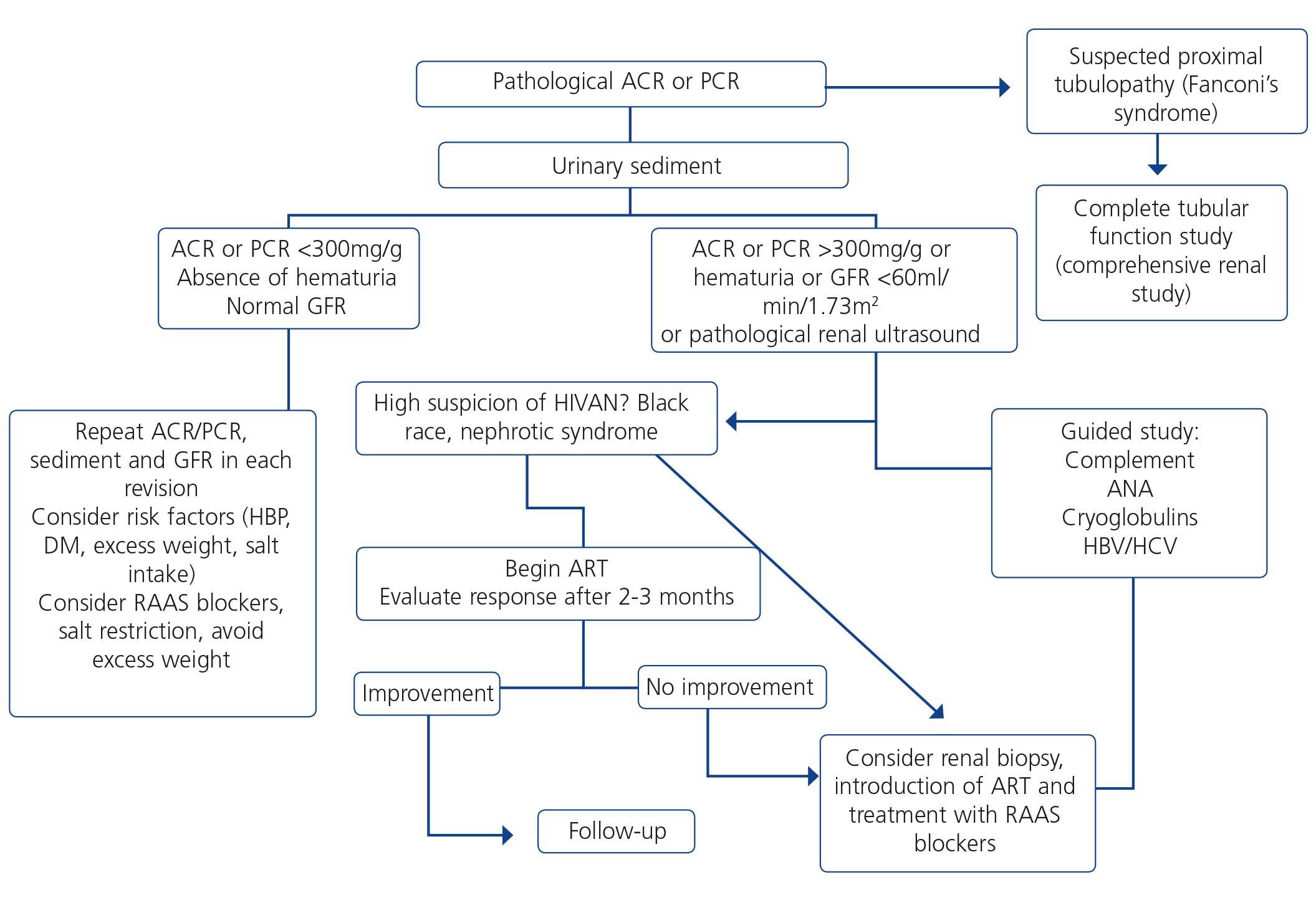
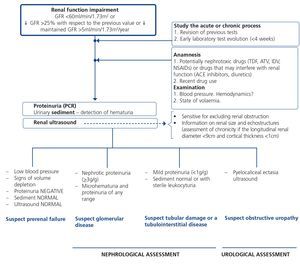
When renal failure is diagnosed, initially it should be determined if the deterioration is temporary and progression should be assessed. The previous serum creatinine values must therefore be reviewed and, in the event that no previous results are available, a renal function test must be repeated within a period no greater than four weeks to assess progression, regression or stability. Following renal failure diagnosis, the aetiological diagnosis must be carried out, assessing whether the cause is prerenal, parenchymal or obstructive by an adequate anamnesis and physical examination, determination of proteinuria (PCR), a study of the urinary sediment (detection of hematuria, leukocyturia, cylindruria) and a renal ultrasound study. In addition to ruling out obstructive urinary processes, the ultrasound allows assessment of the size and echostructure of the kidneys. Small kidneys (usually <9cm in longitudinal diameter) and particularly with a reduction in the thickness of the renal cortex (usually <1cm) and hyperechogenicity of the latter normally indicate chronicity, although HIV-infected patients with CKD do not always have small kidneys. Figure 2 displays a diagram of the process to be followed when there is renal function deterioration.
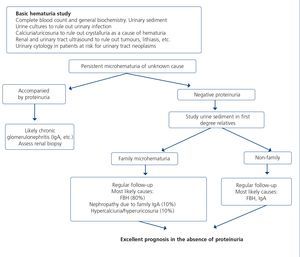
7.1.3. Approach to the presence of hematuria
When hematuria is present, a specific clinical history (history of urinary infection or lithiasis, dysuria, vesical tenesmus, frequent urination, nocturia, prostate symptoms, a family history of hematuria or lithiasis), a complete physical examination, an analysis that includes a complete blood count, serum creatinine concentration and GFR estimation, a urinary sediment study, a urine culture, PCR in urine and a renal ultrasound should be carried out.
The following criteria mean that the cause is probably urological: urinary or prostate symptoms, clots, bright red macroscopic hematuria, the presence of risk factors for urological tumours (age >40 years of age, smoking habit, treatment with cyclophosphamide, use of analgesics, working with chemical materials or paint) or a pathological ultrasound. In these cases, urinary cytologies may be requested and the patient must be referred to Urology, urgently if hematuria is macroscopic.
A glomerular cause will be suspected in the absence of accompanying symptoms, the presence of proteinuria, HBP, renal failure, dark macroscopic hematuria (coluria), red blood cell casts, dysmorphic red blood cells or waxy casts in the sediment or a normal renal ultrasound. In these cases, the patient must be referred to Nephrology, urgently if hematuria is macroscopic or if there is an acute renal function deterioration.
Some drugs, particularly zidovudine (AZT) and to a lesser extent d4T or lamivudine (3TC), may increase the mean corpuscular volume of red blood cells (macrocytosis), in the same manner as has been detected in patients coinfected with HCV152,153. Although no specific studies have analyzed the effect of these abnormalities in red blood cells in urine in the event that the patient has hematuria, and although AZT is not included in current therapeutic regimens, these changes in the size of red blood cells should be borne in mind as a limitation in the interpretation of the mean corpuscular volume in urine in the event that this test is used for the hematuria study.
Figure 3 displays a diagram of the process that is to be followed in the presence of persistent macro- or microscopic hematuria.
7.1.4. Approach to the presence of proteinuria (see section 7.2.2)
• Recommendations if renal function deterioration or hematuria are detected:
1. When there is any renal function deterioration, it is necessary to study whether the process is acute or chronic, observing the changes in previous results (mainly of serum creatinine concentration and GFR estimation). (Quality of the evidence: Low. Recommendation grade: Strong).
2. The following are considered to be progression criteria:
• The change to a more severe CKD stage accompanied by a decrease in the eGFR of >25% with respect to the baseline value.
• A sustained decrease in the GFR >5ml/min/1.73m2/year.
3. In patients with hematuria, a differential diagnosis should be carried out between urological and glomerular causes. (Quality of the evidence: Low. (Recommendation based on consensus).
7.2. Management of chronic kidney disease progression factors, cardiovascular risk factors and other renal abnormalities present in HIV-infected patients with chronic kidney disease
It is known that several variables, including higher CrCl at the time of renal biopsy, a higher CD4 lymphocyte count, an undetectable HIV plasma viral load, the absence of HCV coinfection and/or HCV viremia, the use of ACE inhibitors or ARBs and ART, are factors that have been associated with better renal prognosis and/or higher survival rates in HIV-infected patients diagnosed with CKD by biopsy67,70,80,154.
There is less information about factors that favour progression to end-stage renal disease. A United States Veterans Aging Cohort Study has recently been published in which a database of 22,156 patients without pre-existing renal disease was analyzed. The incidence of end-stage renal disease was 3 cases/1000 individuals-year. The risk factors in this study were those already known: HBP, DM, CVD, CD4 <200 cells/ml, HIV viral load ≥30,000 copies/ml, HCV coinfection and hypoalbuminemia. Furthermore, in this analysis, the combination of a low eGFR and high proteinuria was associated with a higher rate of end-stage renal disease, which reached 193 events/1000 individuals-year for patients with proteinuria ≥300mg/dl and eGFR <30ml/min/1.73m2. These data cannot necessarily be extrapolated to other populations or females, but they highlight the importance of combining the degree of proteinuria with the GFR to stratify the risk of developing end-stage renal disease155.
Table 7 displays the factors and variables that may influence CKD progression in the general population, which would operate in the same manner in HIV-infected patients, as well as the recommended nephroprotective treatment and cardiovascular prevention measures.
We must highlight the importance of controlling proteinuria and HBP156,157 and insist on the prevention and early treatment of episodes of acute renal function deterioration (due to volume depletion and other causes) and of nephrotoxicity, avoiding insofar as possible the use of nephrotoxic substances (NSAIDs, iodinated contrasts, etc.), adjusting the drug dose to renal function, frequently verifying GFR variations when risk medication is used and using ARF prophylactic measures (for example, administration of saline with iodinated contrasts). Below, we describe the approach to the presence of these renal progression and CVR factors in HIV-infected patients.
7.2.1. Cardiovascular risk
CVD is one of the main causes of death not related to HIV infection158. In these patients, there is an increase in CVR associated with factors such as the chronic inflammatory response to HIV infection and with secondary metabolic effects of antiretroviral medication (insulin resistance, dyslipidemia, abnormality in fat distribution and HBP)159. As in the general population, HIV-infected patients have shown an increased life expectancy, which means that they have a higher prevalence of CVR factors such as HBP and DM160. The early diagnosis, treatment and prevention of CVD have become one of the priorities in the care of HIV-infected individuals.
It is recommended to carry out a CVR evaluation during the baseline visit and at least once a year. This evaluation will include:
• CVR factors history (ischemic heart disease, dyslipidemia, HBP, DM, smoking habit, peripheral vascular disease) and risk stratification (Framingham).
• Anthropometric data (weight, height, BP, body mass index, waist and hip circumference and clinical signs of lipodystrophy).
• Laboratory data: lipid study (serum cholesterol concentration, triglycerides, high-density lipoprotein-associated cholesterol [HDL-c] and low-density lipoprotein-associated cholesterol [LDL-c]), glycemia and renal function (serum creatinine concentration and eGFR).
CKD patients have very high CVR. As such, the targets that must be achieved in some risk factors are stricter than in the general population.
• Section 7.2.1 Recommendations:
1. CKD patients are considered to be very high CVR patients. It is recommended to carry out a CVR evaluation in the baseline visit and at least once a year. (Recommendation based on consensus).
2. All CVR will be treated. (Recommendation based on consensus).
3. In diabetic patients, albuminuria should be monitored as an early marker of diabetic nephropathy and HBP. (Quality of the evidence: High. Recommendation grade: Strong).
7.2.2. Proteinuria
We will define moderate asymptomatic proteinuria as that with a PCR of 0.15-1g/g (or its equivalent 0.15-1g/24 hours), significant asymptomatic proteinuria as that with a PCR of 1-3g/g (or its equivalent: between 1 and 3-3.5g/24 hours), provided that it is not accompanied by edema or the biochemical characteristics of the nephrotic syndrome (hypoalbuminemia, hyperlipidemia), and lastly, nephrotic proteinuria if the PCR is greater than 3-3.5g/g (or its equivalent 3-3.5g/24 hours) and is accompanied by edema or hypoalbuminemia, hypoproteinemia or hypercholesterolemia.
It is recommended that patients with a PCR greater than 0.5g/g or those with an ACR greater than 300mg/g be referred to Nephrology.
The approach that is to be followed in patients with proteinuria is displayed in Figure 4. The clinical context and associated markers may suggest the origin. When proteinuria is intense and/or there are abnormalities in the sediment (particularly microhematuria and red blood cell casts), the most likely cause is a glomerular disease. By contrast, when proteinuria is not intense and is accompanied by tubular function abnormalities, the origin is most likely a tubular disease. When there is severe immunosuppression with or without nephrotic syndrome, microhematuria and a decrease in the GFR, a HIVAN or immune complex-mediated nephropathy must be suspected (Table 4). When there is DM and/or HBP, it is most likely that the proteinuria is secondary to diabetic nephropathy or nephroangiosclerosis. In the presence of HBV/HCV coinfection, the main consideration must be a membranous or membranoproliferative GN.
The amount of proteinuria is a decisive factor for the progression of any nephropathy, including those associated with HIV infection. It has been demonstrated that the reduction in proteinuria with blocking of the renin-angiotensin-aldosterone system significantly slows down this progression. The general target is a PCR <0.5g/g (or its equivalent, proteinuria <0.5-1g/24 hours) and in patients with a poor response to antiproteinuric measures, a PCR <1g/g (or its equivalent, proteinuria <1g/24 hours), or at least <50-75% of the baseline value. In nephrotic syndrome, partial remission (PCR <303.5g/g or proteinuria below 3-3.5g/24 hours) may be considered a satisfactory target. The antiproteinuric therapeutic measures are as follows: ACE inhibitors or ARBs (gradually increasing the dose to achieve the proteinuria and BP targets), considering the introduction of an aldosterone antagonist (spironolactone, eplerenone) in cases of poor response to ACE inhibitors/ARBs (in patients with maintained renal function and without hyperkalemia), adequate control of BP values (<130/80mmHg) with the simultaneous use of other antihypertensive drugs if necessary, weight reduction (low-calorie diets, physical exercise) in obese patients and a low-salt diet with moderate protein restriction. Eplerenone may increase its area under the curve, boosting its effect when it is administered with potent CYP3A4 inhibitors (such as RTV or nelfinavir [NFV]). With spironolactone, this interaction does not occur.
• Recommendations for the evaluation and treatment of proteinuria. Section 7.2.2:
1. Whatever the cause of proteinuria, patient management must include the withdrawal of nephrotoxic drugs, the control of HBP and DM (if applicable) and, in any case, if the proteinuria is intense, treatment with renin-angiotensin-aldosterone system blockers (ACE inhibitors or ARBs). (Quality of the evidence: based on consensus).
2. When proteinuria is >1g/24 hours or is accompanied by microhematuria or a GFR <60ml/min/1.73m2, the study should be extended in order to identify the cause. (Quality of the evidence: based on consensus).
3. Antiproteinuric treatment will mainly be based (except in contraindications due to intolerance) on the blocking of the renin-angiotensin-aldosterone system (with ACE inhibitors, ARBs or aldosterone antagonist diuretics, gradually increasing the dose to achieve proteinuria and BP targets) and frequent monitoring of renal function and serum potassium concentration. (Quality of the evidence: High Recommendation grade: Strong).
4. In patients with proteinuria, the therapeutic target is a PCR <0.5g/g (or 50mg/mmol) and in patients with a difficult response to the antiproteinuric measures, a PCR <1g/g (or 100mg/mmol) or at least a reduction of the PCR >50-75% of the baseline value. (Quality of the evidence: Moderate. Recommendation grade: Strong).
5. In patients with nephrotic syndrome, partial remission (PCR below 3-3.5g/g or 3-3.5g/24 hours) may be considered to be a satisfactory target. (Quality of the evidence: Moderate. Recommendation grade: Weak).
6. When a HIVAN is suspected, it is recommended to introduce ART. If there is no improvement in renal abnormalities in a period of two or three months and/or other diagnoses are considered, a renal biopsy should be carried out. (Quality of the evidence: High. Recommendation grade: Strong).
7.2.3. High blood pressure
HBP is present in 80%-90 % of patients with CKD, particularly in advanced stages of renal dysfunction. In large patient cohorts, such as in the Swiss HIV Cohort Study, the prevalence of HBP in the HIV-infected population is 25%, with there being insufficient control of BP in a high percentage161. In a cross-sectional analysis, 55% of patients with HIV and CKD had high blood pressure and 20% were diabetic31. As such, blood pressure should be monitored regularly in HIV-infected patients, particularly if they also have CKD. Blood pressure should be taken in the clinic or measured at home.
The results of controlling BP on progression of renal failure are contradictory. However, BP control is a therapeutic objective, since it significantly decreases mortality and the number of events of cardiovascular origin and it contributes to reducing proteinuria. The BP target is <140/90mmHg in patients without albuminuria/proteinuria (ACR <30mg/g or PCR <0.1g/g) and lower than 130/80mmHg in patients with albuminuria (ACR >3mg/g or PCR >0.1g/g) (see section 7.2.2). Antihypertensive treatment will be adapted to the individual characteristics of each case, although, bearing in mind what was said previously, blocking the renin-angiotensin-aldosterone system will constitute its fundamental basis, particularly in patients with albuminuria/proteinuria. If calcium channel blockers are used, the potential pharmacokinetic interactions must previously be controlled with antiretroviral drugs (see section 7.2.3.3.5).
Although no prospective studies have evaluated the role of these treatments in the CKD of HIV-infected patients, it is considered that the same benefits could be obtained as in the population without HIV infection. Consequently, the recommendations will be the same as those for CKD patients without HIV infection162-164.
7.2.3.1. Blood pressure target values
Although there is controversy about target BP values in CKD patients in the different guidelines, particularly due to a lack of clinical trials, in CKD patients, the target levels are lower than in the general population162:
• Patients with an ACR <30mg/g (or its equivalent, a PCR <0.1g/g or albuminuria <30mg/24 hours) in whom BP values >140/90mmHg are detected must receive treatment to achieve target systolic blood pressure (SBP) ≤140mmHg and diastolic blood pressure (DBP) ≤90mmHg.
• Patients with an ACR >30mg/g (or its equivalent, a PCR >0.1g/g or albuminuria >30mg/24 hours) in whom BP values >130/80mmHg are detected must receive treatment to achieve target SBP ≤130mmHg and DBP ≤80mmHg, since albuminuria is an indicator of renal progression.
7.2.3.2. Non-drug treatment
HBP treatment in CKD patients with HIV infection must include non-drug methods (low-salt diet, intake of <6g salt per day), and avoiding being overweight, avoiding smoking, restricting alcohol intake (no more than two standard drinks per day in males and one in females) and practising aerobic exercise (30 minutes, five times a week).
7.2.3.3. Drug treatment
Antihypertensive treatment in HIV-infected patients will be individualized based on the presence of albuminuria/proteinuria, comorbidities, the effect on some metabolic parameters and concomitant medications that may favour interactions. Table 11 lists the groups of antihypertensive drugs with their possible interactions with ART and their adjustments to renal function.
Both in clinical trials and in daily clinical practice, CKD patients with a decreased GFR usually require an average of 2-3 drugs for controlling HBP.
7.2.3.3.1. Drug group to be used according to the presence of albuminuria/proteinuria
• In patients with an ACR <30mg/g (or its equivalent, a PCR <0.1g/g or albuminuria <30mg in 24-hour urine), there is no evidence supporting the use of one particular drug group.
• In patients with an ACR >30mg/g (or its equivalent, a PCR >0.1g/g or albuminuria >30mg in 24-hour urine), antihypertensive treatment must begin with renin-angiotensin-aldosterone system blockers (ACE inhibitors or ARBs), when BP values indicate it.
• In patients with an ACR >300mg/g (or its equivalent, a PCR >0.3g/g or proteinuria >300mg/24 hours), ACE inhibitors or ARBs will be used in progressive doses to achieve BP ≤130/80mmHg.
7.2.3.3.2. Angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers in the treatment of high blood pressure
ACE inhibitors or ARBs are the drugs of choice if the patient has albuminuria/proteinuria, due to their effect of reducing the latter162. Furthermore, their excellent tolerance, their beneficial effect on the metabolic profile and the practical absence of interactions with ART mean that these are the drugs that must be considered first in HIV-infected patients. The combination of ACE inhibitors and ARBs is not recommended in treating HBP because it is associated with a greater risk of hyperkalemia and renal deterioration, particularly in atheromatous patients, both in diabetics157 and in non-diabetics32,165,166.
7.2.3.3.3. Preventive measures in patients who receive angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers
However, and despite being the drugs of choice, some preventive measures must be taken into account, particularly in relation to renal deterioration and hyperkalemia in patients with risk factors for their development32.
Renal deterioration: the administration of ACE inhibitors or ARBs causes vasodilatation of both the afferent and efferent glomerular arterioles, which is more pronounced in the efferent arterioles. This induces a decrease in intraglomerular pressure and therefore in albuminuria/proteinuria167. In some cases, this effect may cause a decrease in the GFR and an increase in creatinine levels, particularly if they are combined with high doses of diuretics or NSAIDs in a situation of congestive heart failure or advanced renal failure. If the decrease in the GFR or increase in serum creatinine is lower than 25%, it is considered to be attributable to this renoprotective mechanism when it occurs after the introduction of drugs that block the renin-angiotensin system. Decreases in the GFR or increases in serum creatinine greater than 25%, particularly if they are greater than 30%, indicate that there could be an atheromatous disease of both renal arteries (stenosis), provided that functional factors have been ruled out (diarrhoea, vomiting, depletion due to diuretics) (see section 3.1.1). Even if this renal deterioration occurs, once the functional factors have been corrected, ACE inhibitors or ARBs may be reintroduced provided that this deterioration does not occur again and bilateral stenosis of the renal artery has been ruled out. Administration of ACE inhibitors or ARBs will be carried out with precaution if the GFR is <30 ml/min/1.73 m2, due to a greater risk of renal deterioration and hyperkalemia.
Hyperkalemia: the administration of ACE inhibitors or ARBs, and particularly the combination of both, or with NSAIDs or potassium-sparing diuretics, may cause Hyperkalemia. The foregoing combinations are not recommended, and it is preferable to avoid their administration with serum potassium values >5mEq/l.
7.2.3.3.4. Diuretics
As salt retention is an important factor in the pathogeny of HBP in CKD, this drug group will be useful. There are three groups of diuretics: thiazide diuretics (hydrochlorothiazide, chlorthalidone and thiazide-like diuretics such as indapamide), loop diuretics (furosemide, torsemide) and distal diuretics (spironolactone, eplerenone). Thiazide diuretics have a negative effect on glucose metabolism and may exacerbate hyperglycemia and some features of the metabolic syndrome, especially when combined with beta-blockers. By contrast, they have a beneficial effect on osteoporosis by increasing real reabsorption of calcium, increasing mineral density and decreasing the incidence of fractures.
In patients with a GFR <30ml/min/1.73m2, thiazides have a lower natriuretic effect, and as such, it is recommended that loop diuretics (furosemide, torsemide or bumetanide) be used in these patients162. Loop diuretics inhibit tubular calcium reabsorption. The administration of potassium-sparing diuretics (spironolactone, eplerenone, amiloride/triamterene) must be avoided in patients with GFR <30ml/min/1.73m2 due to the risk of hyperkalemia162. They can be indicated as antiproteinuric drugs, but with GFR >45ml/min/1.73m2. None of them have significant interactions with antiretroviral drugs, apart from eplerenone, whose effect may be enhanced when it is administered with potent CYP3A4 inhibitors (such as RTV or NFV).
7.2.3.3.5. Calcium channel blockers
These are metabolized by CYP3A4 and as such, they potentially interact with PIs and non-nucleoside reverse-transcriptase inhibitors (NNRTIs). The interaction with the PIs causes an increase in the response of calcium channel blockers (headaches, low blood pressure peripheral edema and tachycardia); by contrast, there is a lower hypertensive response due to interaction with NRTIs. This has been reported with all calcium channel blockers (amlodipine, nifedipine, felodipine, diltiazem and verapamil) when they were combined with PIs. In some cases, a severe interaction has been reported168,169. LPV/ritonavir (LPV/r) can increase the area under the curve of nifedipine by more than 90%, IDV/ritonavir by 89% and ATV boosted with RTV increases the area under the curve of diltiazem by 125%170. Consequently, if it is necessary to use calcium channel blockers along with PIs, it is recommended to introduce them in low doses, observing the possibility of an excessive antihypertensive effect, and monitoring heart rate in the case of using diltiazem. By contrast, the administration of NNRTIs (efavirenz [EFV] or nevirapine [NVP]) decreases the area under the curve of calcium channel blockers, particularly diltiazem. As such, in these cases, they will be increased to the maximum dose in accordance with the clinical response170. Interactions with integrase inhibitors (INSTI), raltegravir (RAL) and DTG have not been reported.
7.2.3.3.6. Betablockers
Some betablockers (atenolol) are mainly eliminated through the kidneys (>85%), and as such, they require a dose adjustment in patients with a decreased GFR. Adjustment due to renal failure is not necessary in other betablockers. These drugs have a negative effect on the lipid profile and the metabolism of glucose, particularly if they are associated with thiazide diuretics. Furthermore, some PIs must be used with caution or not used in HIV-infected patients, since they interact and cause low blood pressure and blocking of atrioventricular conduction due to a mild increase in betablocker levels in blood, particularly metoprolol and propranolol. If the administration of these drugs is required, it is recommended to monitor heart rate and potential side effects. Significant interactions between antiretrovirals and other betablockers have not been reported170.
7.2.3.3.7. Others
In patients with HBP that has not been controlled with previous drugs, alphablockers (doxazosin), adrenergic agonists (methyldopa, clonidine or moxonidine) or direct vasodilators (hydralazine) may be used. There is less experience with these drugs in HIV-infected patients.
• Recommendations in the management of high blood pressure:
1. Non-pharmacological measures in the treatment of HBP are the same as in the general population: salt restriction (<6g/day), control of excess weight, physical exercise (at least walking at a brisk pace 30-45 minutes, four days a week). (Quality of the evidence: High. Recommendation grade: Weak).
2. The antihypertensive treatment target will depend on the presence or absence of albuminuria/proteinuria. (Quality of the evidence: High. Recommendation grade: Strong).
3. In patients with an ACR <30mg/g (or its equivalent, a PCR <0.1g/g), the BP target will be <140/90mmHg. (Quality of the evidence: High. Recommendation grade: Strong).
4. In patients with an ACR >30mg/g (or its equivalent, a PCR >0.1g/g), the BP target will be <130/80mmHg. (Quality of the evidence: High. Recommendation grade: Weak).
5. In the presence of albuminuria/proteinuria, antihypertensive treatment must begin with renin-angiotensin-aldosterone system blockers (ACE inhibitors or ARBs), when BP values indicate it. In patients with an ACR <30mg/g (or its equivalent, a PCR <0.1g/g), there is no evidence that supports the use of any particular drug group. (Quality of the evidence: High. Recommendation grade: Strong).
6. Calcium channel blockers must be used with caution in patients who receive HIV PIs (they increase their antihypertensive effect) and NNRTIs (they decrease their antihypertensive effect), due to the possibility of drug interaction. If calcium channel blockers are required, RAL/DTG-based regimens are recommended. (Recommendation based on consensus).
The treatment of HBP must follow the steps below:
• First step: ACE inhibitors or ARBs.
• Second step: diuretics:
-Low or medium doses of thiazide diuretics (equivalent to 12.5-25mg of hydrochlorothiazide) with mild to moderately impaired renal function (serum creatinine concentration of up to 2.0mg/dl or a GFR >30ml/min/1.73m2).
-Loop diuretic with moderate-advanced renal failure (GFR <30ml/min/1.73m2).
• Third step: betablockers (avoid calcium channel blockers if the patient is receiving treatment with PIs).
7.2.4. Smoking habit
Some research suggests that smoking may favour CKD progression, that heavy smokers have a greater risk of renal disease and that quitting smoking may be associated with a reduced rate of renal failure progression171, and thus the avoidance of smoking has been recommended in CKD patients.
7.2.5. Obesity
The influence of obesity (body mass index >30kg/m2) and even being overweight (body mass index >25kg/m2) on the progression to end-stage renal disease of various nephropathies has been shown in various studies172,173. Obesity itself is a recognised cause of renal disease (glomerular disease associated with obesity) and it is the cause of DM. The treatment will consist of a low-calorie diet.
7.2.6. Metabolic acidosis
In controlled prospective studies, it has been observed that treatment with bicarbonate slows down progression in patients with CKD and associated metabolic acidosis174. Although the therapeutic target is not yet clear in terms of pH and serum bicarbonate levels, the presence of metabolic acidosis must be monitored in patients with renal disease.
7.2.7. Hyperuricemia
Recent research suggests that treatment with allopurinol reduces the progression of renal damage, BP and cardiovascular events. Although we require more comprehensive studies that corroborate these findings and set therapeutic targets in relation to uric acid concentration, we recommend monitoring these values and indicating dietary (low-purine diet) and drug (allopurinol) measures in refractory cases, when there is gout and probably when serum concentration of uric acid is very high (>9-10mg/dl)175. Although there is no experience in HIV-infected patients, in those with CKD not infected with HIV, the administration of febuxostat has shown to be more effective and have a similar safety to allopurinol in symptomatic hyperuricemia and mild or moderate renal failure151. However, and given that febuxostat is metabolized in the liver by CYP450 and glucuronidation, there could be drug interaction with certain antiretroviral drugs.
7.2.8. Acute renal failure episodes
Patients who have suffered an episode of ARF of any aetiology have a higher risk of developing CKD. Therefore, the renal function of these patients must be monitored more frequently for at least two years after ARF, even if it resolved satisfactorily.
7.2.9. Race
Recent studies have demonstrated the relationship between genetic mutations in the APOL 1 gene and the predisposition of Black patients to develop focal glomerular sclerosis, refractory HBP and HIVAN176. There should be close follow-up and control of potential renal abnormalities in Black HIV-infected patients.
7.2.10. Protein restriction
Although various studies have demonstrated the usefulness of protein restriction in delaying CKD progression177, the difficulty in achieving it, the risk of malnutrition and the modest benefits expected from this measure mean that its role in HIV-infected patients is questionable and, in any case, restricted to patients with moderate or severe CKD under strict dietary supervision.
7.2.11. Coinfection with the hepatitis C virus
(see section 8)
• Recommendations in the approach to other risk factors (obesity, smoking habit, other):
1. The prevention and treatment of obesity and excess weight is recommended, given their recognized association with the progression of renal failure. (Quality of the evidence: Low. Recommendation grade: Strong).
2. Quitting smoking must be a priority, given that, as well as its influence on many complications, it influences the progression of renal failure. (Quality of the evidence: Low. Recommendation grade: Strong).
3. It is recommended to monitor the presence of metabolic acidosis and, if it is severe and progressive, introduce treatment with bicarbonate orally, as with non-infected patients. (Quality of the evidence: Low. Recommendation based on consensus).
4. It is recommended to monitor serum uric acid concentration, indicating dietary measures in cases of moderate hyperuricemia and adding hypouricemic drugs in refractory cases or those with very high urate concentrations. (Quality of the evidence: Low. Recommendation grade: Weak).
7.3. Management of dyslipidemia in HIV-infected patients with chronic kidney disease
HIV-infected patients very often have lipid metabolism disorders that contribute to increasing their CVR. These disorders are hypertriglyceridemia and a reduction of HDL-c178.
7.3.1. Lipid pattern in chronic kidney disease
CKD is associated with quantitative and qualitative abnormalities in the lipid pattern, which are accentuated as the disease becomes more severe. The typical lipid pattern consists of an increase in triglyceride concentration with a decrease in HDL-c, while the increase in total cholesterol and LDL-c is less marked in stages 1 and 2. There is a predominance of small and dense LDL-c particles, which leads to a greater risk of atherothrombosis. Dyslipidemia in CKD has similar characteristics to those of HIV-infected patients, and as such, HIV-infected patients who develop CKD probably have more pronounced dyslipidemia than in the case of each of the diseases separately. When there is dyslipidemia in CKD patients, it is important to rule out secondary causes (nephrotic syndrome, hypothyroidism, DM, liver disease, alcoholism and some pharmaceutical drugs) (Table 12).
Control of dyslipidemia associated with renal disease is a therapeutic objective due to its proven protective effect on cardiovascular complications. The targets will be those set out by the guidelines for patients with high CVR179.
7.3.2. Evidence of the benefit of treating dyslipidemia in chronic kidney disease
Data obtained from post hoc analyses support the ability of statins to reduce cardiovascular complications in patients with stages 2 and 3 CKD180. Given the results obtained in clinical trials181-183, it is not recommended to begin treatment with statins in dialysis patients179, because it is considered too late to obtain some benefit that overcome the risks of adverse events. However, the results of the SHARP study showed a 17% reduction in cardiovascular events in subjects with stages 3, 4 and 5 CKD treated with simvastatin-ezetimibe versus placebo. This reduction was not observed in dialysis patients183.
7.3.3. Therapeutic objectives
In 2003, the National Kidney Foundation Kidney/Disease Outcomes Quality Initiative (K/DOQI) published consensus guidelines for managing dyslipidemia in patients with renal failure, which have recently been revised184. This working group concluded that in patients with CKD, the interventions recommended by the National Cholesterol Education Program (NCEP ATP-III) expert committee should be taken, since they are expected to reduce the risk of CVD of arteriosclerotic origin in a similar manner as in the general population. The risk of cardiovascular events after 10 years in CKD patients is less than 20%, and as such, it is recommended to apply the most stringent NCEP ATP-III targets in terms of lipid reduction. The latest European Atherosclerosis Society and European Society of Cardiology guidelines consider HIV-infected patients to be high CVR patients, and theoretically, the concentration of LDL-c that it would be necessary to achieve would be 100mg/dl (2.5mmol/l). However, the same guidelines consider that the presence of CKD with a GFR <60ml/min/1.73m2 classifies the patient as having very high CVR, without the risk scales being necessary, and it advises the implementation of even more aggressive therapeutic objectives179.
7.3.3.1. Therapeutic objective for low density lipoprotein-associated cholesterol
The reduction of LDL-c is advised as a primary objective. CKD has an equivalent CVR to ischemic heart disease and the target that must be achieved in patients with a GFR <60ml/min/1.73m2 for LDL-c is <70mg/dl (<1.8mmol/l) or a 50% reduction if this target cannot be achieved179. The drugs of choice are statins, alone or associated with ezetimibe.
However, the KDIGO guidelines for treating dyslipidemia in CKD have recently recommended statin treatment for all adults >50 years of age, regardless of LDL-c levels. Based on the SHARP study and in a post hoc analysis of clinical trials with statins versus placebo in CKD patients, a strategy to “treat CVR” was established without considering LDL-c targets. Similarly, they did not recommend introducing treatment with statins in stage 5 CKD patients on dialysis185.
Due to the absence of specific guidelines for treating dyslipidemia, it has always been advised to follow the guidelines for CKD patients without HIV infection, and as such, the recommendations will be based on the European Atherosclerosis Society and European Society of Cardiology179 guidelines, since the very recently published KDIGO guidelines may be a source of controversy and it is appropriate to wait to know their impact on scientific societies and o• Recommendations for CKD patients.
7.3.3.2. Therapeutic objective in hypertriglyceridemia
Adults with any stage of CKD who have hypertriglyceridemia, when secondary causes have been ruled out, are recommended to make lifestyle changes (diet, physical activity and abstaining from alcohol consumption). Drug treatment of hypertriglyceridemia with fibrates is advised if the triglyceride concentration is >500mg/dl (>5.6mmol/l) in order to prevent the onset of pancreatitis179. Although in the general population it is desirable to achieve a triglyceride level ≤150mg/dl (<1.7mmol/l), it is advised to start drug treatment to decrease triglycerides in high-risk patients with triglyceride values >200mg/dl (>2.26mmol/l) when the change in lifestyle has not been effective. However, there is no evidence that treatment with fibrates is associated with an improved CVR in CKD patients, and on the contrary, they are related to a higher frequency of adverse effects, particularly if they are combined with statins. Consequently, treatment with fibrates is not recommended in CKD patients, unless the level is higher than 800-1000mg/dl179,185.
7.3.4. Lipid-lowering drugs and chronic kidney disease
• Statins: drugs that are mainly eliminated by the liver (atorvastatin and pitavastatin) are considered to be the drugs of choice. Statins metabolized via CYP3A4 (atorvastatin, lovastatin, simvastatin) or CYP2C9 (rosuvastatin) may increase side effects by boosting drug interactions when they are administered with certain inducers or inhibitors. The maximum rosuvastatin dose in CKD is 10mg/day. Except for pravastatin, all statins are metabolized by the cytochrome P450 enzyme complex (CYP), in particular the isoenzyme CYP3A4, which metabolises atorvastatin, lovastatin and simvastatin, which may lead to a series of interactions with other medications that are regularly used in these patients (cyclosporine, tacrolimus, azole antifungals, macrolides, fibrates, nicotinic acid derivatives, PIs and oral anticoagulants). Fluvastatin, on the other hand, is metabolized by the CYP2C9 route and there are fewer interactions, although it is necessary to adjust their dose when they are administered with NNRTIs. Pitavastatin is metabolized in the liver by a different route than that of CYP450, and as such, it is particularly useful in renal transplantation due to the absence of interactions with immunosuppressants and antiretroviral drugs. For these reasons, statins in CKD patients with HIV infection will be selected based on their metabolization (CYP3A4, CYP2C9), their interaction with each antiretroviral drug and the degree of renal failure and maximum dose of each statin. In summary, pitavastatin, atorvastatin, rosuvastatin and pravastatin would be recommended, adjusted to the degree of renal failure (Table 13).
• Ezetimibe: it has shown its efficacy in combination with simvastatin in CKD patients in the SHARP study183. No dose adjustment is required in renal failure (Table 13).
• Fibrates: if fibrates are used, the drug of choice is gemfibrozil in doses of 600mg/day provided that they are not combined with statins, in which case the risk of myopathy increases more than five times, and it is higher in the case of CKD. The combination with statins may cause rhabdomyolysis-induced ARF. If their combination is necessary, fenofibrate must be used and the situation must be closely monitored. They are not recommended in patients with a GFR <50ml/min/1.73m2 (Table 13).
Fibrates increase creatinine blood concentration, which is reversible when they are withdrawn. In the case of fenofibrate, the increase varies between 8% and 18%. The mechanisms involved are not entirely clear136,137 but the increase in creatinine is not associated with an increase in its production, and as such, it is suggested that it acts by reducing the GFR through glomerular hemodynamic mechanisms186.
Furthermore, fibrates increase cyclosporine clearance, leading to a 30% reduction in its levels187. Fibrate use is not advised in renal transplantation.
• Nicotinic acid: nicotinic acid has not been studied in CKD patients, and as such, its use is not advisable, given the high prevalence of side effects (redness and hyperglycemia). Furthermore, the Spanish Agency of Medicines and Medical Devices has more recently reported a higher frequency of some types of severe adverse reaction to nicotinic acid188, and as such, it is not recommended in HIV-infected patients.
• Section 7.3 recommendations:
1. In patients with CKD and HIV infection, a lipid study is recommended (which includes the measuring of serum cholesterol, triglycerides and HDL-c and LDL-c concentration) every six months. (Recommendation based on consensus).
2. The control of dyslipidemia in the context of renal disease is a therapeutic objective, given its proven protective effect on cardiovascular complications. The targets will be those set by the guidelines for patients with high CVR. (Quality of the evidence: High. Recommendation grade: Strong).
3. Patients with CKD and HIV infection with a GFR <60ml/min/1.73m2 are considered to have very high CVR, without the need to apply risk scales. The therapeutic objective is a LDL-c concentration <70mg/dl or a 50% reduction if this target cannot be achieved. The treatment will include a low-fat diet and drug treatment with statins, in combination or not with ezetimibe. (Quality of the evidence: Moderate. Recommendation grade: Weak).
4. The choice of statins in CKD patients with HIV infection will depend on their metabolization (CYP3A4 CYP2C9), their interaction with each antiretroviral drug and the degree of renal failure and maximum dose of each statin. Pitavastatin, atorvastatin, rosuvastatin and pravastatin would be recommended, adjusted to the degree of renal failure. (Recommendation based on consensus).
5. If hypertriglyceridemia is detected, lifestyle changes and drug treatment with fibrates are recommended if the triglyceride level is >800-1000mg/dl with the aim of preventing the onset of pancreatitis. If fibrates are used in combination with statins, fenofibrate must be used and must always be adjusted to the degree of renal failure, and the possibility of rhabdomyolysis and renal function deterioration, which is usually reversible, must be monitored. (Quality of the evidence: Moderate. Recommendation grade: Weak).
6. Combined treatment with statins and fibrates is not advised in renal transplantation. (Recommendation based on consensus).
7.4. Management of hyperglycemia in HIV-infected patients with chronic kidney disease
Type 2 DM is a growing problem among the HIV-infected population. In the study by Galli et al.189, the prevalence of type 2 DM in HIV-infected patients was almost twice as high as in healthy subjects of the same age and sex. Although HIV is not involved in the pathogenesis of DM, ART may influence the onset of DM through various mechanisms. The first is through the wellbeing produced by ART, which is associated with weight gain, an improved appetite and calorie intake. Furthermore, some antiretroviral drugs have an effect on glucose metabolism, by increasing insulin resistance or impairing its secretion, thus favouring hyperglycemia. While d4T and ddi are associated with a greater risk of DM, RTV and NVP appear to be protective in this regard190.
DM has been associated with risk factors typical of the general population, a longer duration of HIV infection and the lowest CD4 nadir189. DM in an HIV-infected patient will increase the risk of renal involvement due to the potential development of diabetic nephropathy. The early detection of diabetic nephropathy (regular ACR measurements) and its treatment with antiproteinuric measures and BP control, summarized in the previous sections, are vital, along with adequate control and treatment of DM.
The diagnosis of type 2 DM in HIV-infected patients will be based on the same criteria as in the general population191. There are no randomized studies in the treatment of hyperglycemia in CKD patients with HIV infection. Consequently, the recommendation will be based o• Recommendations for CKD patients without HIV infection (Figure 5 and Table 14).
7.4.1. Management objectives
7.4.1.1. Assessment of metabolic control
Glycated hemoglobin A1c (HbA1c) is the reference parameter for assessing metabolic control in CKD patients, but we should bear in mind that in CKD, there are circumstances that determine its accuracy. Uremia favours the formation of carbamylated Hb, which interferes in the measurement of HbA1c by high-performance liquid chromatography, leading to falsely high concentrations191. Due to all of these factors, some authors suggest using glycated albumin (fructosamine) as a method of assessing glucose control in hemodialysis patients191. However, this stance is not accepted by the majority.
7.4.1.2. Glucose control targets
Most studies that have assessed glucose control through HbA1c did not stratify patients in accordance with their GFR or CrCl; at best, the renal condition was assessed by serum creatinine concentration. In most patients with short-term DM and CKD, without any condition that decreases their life expectancy, the target must be HbA1c <7%. In patients with CKD and long-term DM with a history of poor glucose control or conditions that decrease their life expectancy, the control target must be individualized, avoiding therapeutic strategies that involve an unacceptable increase in the risk of hypoglycemia. Furthermore, the CVR profile tells us that most patients with CKD stages 4 and 5 belong to the second group. Indeed, the 2005 K/DOQI guidelines192 have not established an optimal HbA1c level for dialysis patients. The most recent recommendations in these patients suggest achieving moderate glucose control with HbA1c concentrations no higher than 8% and avoiding hypoglycemia191.
7.4.2. Oral anti-diabetic drugs
There is little experience with oral anti-diabetic drugs in HIV-infected patients, except for metformin and sulphonylureas. The recommendations in relation to other anti-diabetic drugs are made by extrapolating the data and recommendations in CKD patients who are not infected by HIV and are based on the absence of interactions or other side effects reported in publications to date with this type of treatment in HIV-infected patients193.
•Secretagogues: sulphonylureas are mainly eliminated through the kidney, except for those that are most used (glibenclamide, glimepiride and gliclazide), which are metabolized in the liver to weaker metabolites that are eliminated in urine. As such, its use, even in low doses is not advisable in CKD patients and it is completely contraindicated in patients with a GFR <60ml/min/1.73m2. Glipizide may be used with a GFR194,195 up to <30ml/min/1.73m2. Two studies recently compared glipizide with sitagliptin in CKD patients on hemodialysis. Both drugs were effective in reducing glycemia and were safe. However, patients who received glipizide had more hypoglycemia196,197.
• Metformin: metformin is mainly eliminated in urine without being metabolized. As such, patients with a deterioration of renal function are more susceptible to its accumulation and lactic acidosis. According to the drug’s data sheet, its use is not recommended in patients with CrCl <60ml/min. However, the experience of its use in CKD patients has increased progressively. Indeed, in patients with a GFR of between 45 and 60ml/min/1.73m2, it is permitted and it is advised to monitor renal function every 3-6 months. When there is a GFR between 30 and 45ml/min/1.73m2, it is advised to reduce the dose by 50%, monitor renal function every three months and not nd not introduce it as a new treatment. With a GFR below 30ml/min/1.73m2 metformin195 must not be used193,195,198.
• Glitazones: since they are metabolized in the liver, there is no accumulation of active metabolites in CKD and the pharmacokinetics of pioglitazone are not affected by renal failure or hemodialysis. However, since its use increases the risk of edema and heart failure (particularly in patients treated with insulin) and osteoporosis and distal fractures in postmenopausal women, its use is limited in these patients and it is contraindicated in patients with a GFR <30ml/min/1.73m2.
• Meglitinides (repaglinide): is eliminated via bile, and as such, its use is accepted in any stage of CKD. Nevertheless, treatment should begin with a low dose (0.5mg) and caution should be taken if the GFR is <30ml/min/1.73m2. Nateglinide, despite being metabolized by the liver, is broken down to active metabolites, which are cleared by the kidneys, and as such, it is not recommended in CKD194,195.
• Alpha-glucosidase inhibitors: both acarbose and miglitol and their metabolites are accumulated in CKD, therefore, although it has not been documented that they increase the risk of hypoglycemia, their use is not recommended, probably due to a lack of studies.
• Dipeptidyl peptidase-4 inhibitors: no gliptin requires adjustment with a GFR greater than 50ml/min/1.73m2. Sitagliptin, vildagliptin and saxagliptin require an adjustment of their dose when the GFR is lower than 50ml/min/1.73m2. Sitagliptin must be used in doses of 50mg and 25mg when the GFR is between 50 and 30ml/min/1.73m2 and below 30ml/min/1.73m2, respectively. Vildagliptin must be used in doses of 50mg below 50ml/min/1.73m2, including in end-stage renal disease requiring dialysis. Saxagliptin must be administered in doses of 2.5mg in patients with a GFR below 50ml/min/1.73m2 and its use is not indicated in patients with advanced CKD or on dialysis. Linagliptin does not require a dose adjustment in any CKD stage.
• Glucagon-like peptide-1 agonists (exenatide, liraglutide): effectively reduce glycemia with few or no cases of hypoglycemia and they also cause weight-loss. As such, they are a useful alternative in obese patients (body mass index >35kg/m2). They are not indicated in patients with a GFR <60ml/min/1.73m2.
•Sodium–glucose cotransporter 2 inhibitors: this drug group inhibits glucose reabsorption in the proximal tubule, inducing glucosuria and natriuresis. This group includes: empagliflozin, dapagliflozin, canagliflozin, which will be marketed in 2014. Therefore, there is no experience in HIV-infected patients, since they have not been included in clinical trials. The efficacy of these drugs depends on renal function, and as such, it is lower in patients with moderate CKD and is practically nil in advanced CKD. They are not indicated in patients with an eGFR <60ml/min/1.73m2.
• Insulin: insulin, along with metformin and sulphonylureas, are the treatments with which there is most experience in HIV-infected patients. CKD is associated with insulin resistance, but advanced CKD causes a decrease in insulin degradation, with the resulting reduction in the need for it, and even its discontinuation. Dialysis partially reverses both insulin resistance and the increase in its degradation. Furthermore, we should bear in mind that in patients who receive treatment with peritoneal dialysis, peritoneal fluid has a very high glucose content due to its high content in replacement fluid. As a result, insulin requirements in a certain patient will depend on the balance between improved insulin sensitivity and the normalization of liver metabolism of insulin, and as such, the individualization of insulin treatment is essential. Initial standards that must be adapted to each patient by monitoring glucose include:
GFR >50ml/min/1.73m2: no insulin dose adjustments are required.
GFR: 50-10ml/min/1.73m2: reduce to 75% of the baseline dose.
GFR <10ml/min/1.73m2 reduce to 50% of the dose of CKD not on dialysis.
Close monitoring (glycemia-visits).
The insulin regimen will be adapted to the control target and may be a conventional therapy or an intensive treatment.
• Section 7.4 recommendations:
1. The HbA1c target in CKD patients with HIV infection will be the same as in the general population. In advanced CKD patients (GFR <30ml/min/1.73m2) on dialysis, the HbA1c target will be <8%. Therapeutic strategies will be implemented in order to avoid episodes of hypoglycemia. (Recommendation based on consensus).
2. The use of oral hypoglycemic agents (metformin, sulfonylureas and dipeptidyl peptidase-4 inhibitors) and insulin in CKD patients with HIV infection will be adjusted to the degree of renal function and will follow the same recommendations as for the population without HIV. (Recommendation based on consensus).
7.5. Management of mineral and bone metabolism disorders
Mineral and bone metabolism disorders in CKD is a complex set of pathologies involving disorders of calcium, phosphorus, parathyroid hormone (PTH), vitamin D and phosphaturic factors, causing remodelling, mineralization and volume abnormalities, abnormalities in the growth or fragility of the skeleton and vascular calcifications or calcifications of other soft tissues199. Hyperparathyroidism secondary to CKD is not only associated with renal function deterioration, but in patients with HIV infection they are also involved in vitamin D deficiency, treatment with some antiretroviral drugs and renal loss of phosphate due to tubular toxicity200,201. This situation of secondary hyperparathyroidism may exacerbate the risk of fractures and other bone complications present in HIV-infected patients. Various mechanisms influence the pathogenesis of secondary hyperparathyroidism (increase in intact PTH [iPTH]): increase in serum phosphate concentration, increase in fibroblast growth factor-23, decrease in calcitriol (1,25-(OH)2D3) synthesis (secondary to loss of renal mass) and less tubular absorption of 25-(OH)D3, in which megalin participates, as well as a lower absorption of calcium by the intestine199,202.
Although it has not been proven that correcting these disorders decreases the rate of renal failure progression or prevents renal events, mineral and bone metabolism laboratory parameters should be monitored and their abnormalities should be treated in accordance with the therapeutic guidelines, particularly in patients with advanced renal failure.
25-OH vitamin D (D2 and D3) deficiency is very common, it affects 76%-86% of HIV patients203-205. The risk of deficiency has been associated with ART, but it is much more evident with EFV206. Various mechanisms have been suggested to explain how EFV is associated with a greater vitamin D deficiency. EFV induces cytochromes CYP3A4 and CYP24. With the first induction, it decreases the quantity of substrate (vitamin D3) available for its transformation to 25-OH vitamin D. Moreover, CYP24 increases the destruction of calcidiol [25 (OH) vitamin D] and calcitriol [1,25 (OH)2 vitamin D]. Lastly, the induction by EFV of CYP2R1 reduces the synthesis of 25 (OH) vitamin D205,206. Some authors, although much less frequently, have also reported a greater vitamin D deficiency in patients who receive PIs, since these drugs inhibit 1-alpha-hydroxylase and 25-alpha-hydroxylase, reducing 25-OH vitamin D conversion to active metabolites205.
The context of vitamin D deficiency may the management of mineral and bone metabolism disorders in CKD patients with HIV infection more complicated.
All of the above contributes to skeletal resistance to PTH action. These disorders are more marked as renal function decreases and they are more severe in dialysis patients.
There are few studies on mineral and bone metabolism disorders in the different stages of CKD and this dearth of studies is even more pronounced in HIV-infected patients. Consequently, most recommendations are established as opinions and are extrapolated from guidelines for dialysis patients without HIV infection, in whom there are different grades of evidence.
The recommendations are mainly based on guidelines on CKD patients199,202. In general, the nephrologist will be in charge of managing dialysis patients.
1. In HIV-infected patients with stages 3-4 CKD not on dialysis:
• Correction of 25-OH vitamin D concentration: there is no specific evidence in HIV-infected patients with CKD. Native vitamin D supplements are recommended (cholecalciferol or calcifediol) to achieve serum concentrations of 20-40ng/ml. In patients with CKD and osteoporosis and/or a high risk of fracture, in accordance with the criteria of the World Health Organization, similar treatment to that of the general population is advised. The effect of EFV will be taken into account in patients with a severe 25 OH vitamin D deficiency.
• Phosphate: maintenance of normal phosphate concentration (2.4-4.6mg/dl, 0.8-1.4mmol/l) through diet and calcium and non-calcium binders and maintenance of an efficient dialysis regimen.
• PTH: maintain in recommended ranges in accordance with K/DOQI guidelines (35-70pg/ml in stage 3 CKD and 70-110pg/ml in stage 4 CKD). For its control, active vitamin D (calcitriol) or selective vitamin D receptor activators (paricalcitol) will be administered, preferentially the latter, since they have shown a lower incidence of hypercalcemia, hyperphosphatemia and hypercalciuria199. These treatments will be applied once concentrations of serum calcium, phosphate and the adjustment of calcidiol have been normalized. They should never be administered if phosphate or calcium concentrations are higher than their upper normal ranges.
2. In patients with HIV infection and stage 5 CKD not on dialysis or stage 5 on dialysis:
• Normalization of 25-OH vitamin D levels: the same as with CKD not on dialysis.
• Phosphate: maintenance of normal phosphate concentration (2.4-4.6mg/dl, 0.8-1.4mmol/l) by diet and calcium and non-calcium binders as well as maintenance of an efficient dialysis regimen.
• Calcium: must be maintained within the normal range (8.4-9.5mg/dl or 2.2-2.55mmol/l). The regimen of calcium in dialysate play an important role in this regard.
• PTH: maintaining iPTH levels of 150-300pg/ml, avoiding concentrations lower than 100 and higher than 500pg/ml. For its control, active vitamin D (calcitriol) or selective vitamin D receptor activators (paricalcitol) or calcimimetics (cinacalcet) will be administered, particularly the latter in the case of associated hypercalcemia. These treatments will be administered once serum calcium, phosphate and calcidiol concentrations have been controlled. They will never be administered if phosphate or calcium are above their upper normal ranges.
Given the complexity of coexisting bone lesions in stages 4-5D CKD patients due to mineral and bone metabolism disorders, in patients with a low bone mass density and/or fractures due to bone fragility, we suggest additional studies, and a bone biopsy may be considered before deciding the treatment with antiresorptive medication199.
7.5.1. Hypophosphatemia
In general, CKD is associated with hyperphosphatemia. Hyperphosphatemia does not usually appear in patients with CKD unless they have a major vitamin D deficiency. However, it is an important and relatively common problem in HIV-infected patients and is detected in up to 10% of patients without ART and in 20%-30% of those who receive ART207,208. As such, it is included in mineral and bone metabolism disorders due to the implications that it has on bone209.
Hypophosphatemia is caused by a decrease in phosphate absorption, a vitamin D deficiency, an extracellular redistribution or greater excretion of phosphate in urine. In the latter case, some antiretroviral drugs play an important role, particularly TDF due to its pathogenesis in the development of proximal tubulopathy. Some antiretroviral drugs may have an effect on vitamin D metabolism and influence its level in blood and, indirectly, levels of phosphate in blood203,206.
Table 15 summarises the main causes of Hypophosphatemia.
In accordance with plasma phosphorus levels, Hypophosphatemia is classified as:
• Mild (0.65-0.80mmol/l [2-2.5mg/dl]).
• Moderate (0.32-0.64mmol/l [1-1.99mg/dl].
• Severe (<0.32mmol/l [<1mg/dl]).
Mild and moderate hypophosphatemia usually occurs without symptoms, although depending on the stage of the clinical course, it may present as fatigue, muscle weakness or diffuse bone pain, which is sometimes quite debilitating and a reflection of osteomalacia and bone demineralization lesions. Severe hypophosphatemia, on the other hand, is considered a medical emergency due to the serious consequences and the fatal risk that it carries210.
It is important to bear in mind that phosphate tests must be carried out during fasting and there should be no hemolysis. To achieve a correct diagnosis, it is first necessary to distinguish between a real phosphate deficiency and an isolated and temporary decrease in serum concentrations, normally due to intra- and extracellular redistribution. In series in which the reproducibility of phosphate concentrations in blood was analyzed in serial laboratory tests without therapeutic intervention, it was observed that in 11% of cases hypophosphatemia was not confirmed or spontaneously reversed207.
Once it has been confirmed that the hypophosphatemia is real and constant, the clinical context of the patient must always be assessed, focussing on factors such as alcoholism, severe malnutrition, chronic diarrhoea and particularly drugs (antiretroviral and non-antiretroviral drugs) which they are taking or have taken. The GFR should be estimated and levels of sodium, potassium, urate, bicarbonate, glucose, iPTH and vitamin D in blood, glucosuria, proteinuria and FEP should be tested (see section 4.4).
Along with hypophosphatemia, the presence of hypouricemia, hypokalemia, proteinuria, glucosuria with normal glycemia or renal failure and increased FEP indicates the diagnosis of Fanconi’s syndrome. If hypophosphatemia is not accompanied by any of the abovementioned abnormalities and iPTH is high with normal vitamin D concentrations, we should consider the diagnosis of primary hyperparathyroidism; if vitamin D is low and iPTH is normal or slightly increased, we should consider the diagnosis of vitamin D deficiency or osteomalacia. Low urinary excretion of phosphate indicates lower absorption or a redistribution of phosphate and suggests that the tubule is not damaged, while a high urinary excretion of phosphate suggests that the cause may be a vitamin D deficiency, hyperparathyroidism (generally primary or secondary to CKD with a major vitamin D deficiency) or proximal tubulopathy induced by ART, particularly TDF203,211. The approach to be taken in patients with hypophosphatemia is shown in a diagram in Figure 6.
• Recommendations for mineral and bone metabolism disorders:
1. In patients with a GFR lower than 60ml/min/1.73m2 and HIV infection, concentrations of serum calcium, phosphate and calcidiol (25-OH vitamin D) and plasma iPTH will be tested at least once a year. (Quality of the evidence: Low. Recommendation grade: Weak).
2. Treatment of mineral and bone metabolism disorders in CKD patients with HIV infection will be identical to that of patients not infected with HIV: maintaining serum phosphate concentration within the reference range, avoiding calcidiol (25-OH vitamin D) deficiency and correcting iPTH concentration if it is above the reference range. (Recommendation based on consensus).
3. When serum iPTH concentration is above the recommended limit, the previously mentioned factors will be corrected and treatment with active vitamin D (calcitriol or selective vitamin D receptor activators [paricalcitol]) will be introduced, preferably the latter because they have a lower incidence of hypercalcemia, hyperphosphatemia and hypercalciuria. (Recommendation based on consensus).
4. Hypophosphatemia must always be confirmed with more than one test. (Recommendation based on consensus).
5. Other renal markers of proximal tubulopathy must be studied, in particular the presence of proteinuria and glucosuria and plasma concentrations of potassium, urate and bicarbonate. If there are no abnormalities in other tubular dysfunction markers, hormones regulating calcium and phosphorus metabolism must be analyzed: iPTH and vitamin D in blood. (Recommendation based on consensus).
6. Severe hypophosphatemia (<1mg/dl) requires immediate action, in some cases with the intravenous administration of phosphorus. (Quality of the evidence: High. Recommendation grade: Weak).
7. Mild or moderate hypophosphatemia may resolve with treatment of the aetiology (vitamin D in the case of vitamin D deficiency, surgical resection in primary hyperparathyroidism and withdrawal of the drug or toxin in cases of proximal tubular dysfunction secondary to nephrotoxic drugs). (Quality of the evidence: Low. Recommendation grade: Weak). In some cases, oral phosphate may be used. (Recommendation based on consensus).
7.6. Management of anemia in HIV-infected patients with chronic kidney disease (diagnosis, evaluation and criteria for the administration of iron and erythropoiesis-stimulating agents)
The prevalence of anemia and its intensity may be greater in HIV-infected patients with CKD than in those who are not infected. The direct effect of the virus on erythroid precursors, the presence of opportunistic infections and ART, among others, are factors that may favour anemia. In some studies, it has been observed that anemia may be an independent predictor of worse survival in the HIV-infected population, and as such, these patients must be treated accordingly28,212,213.
In the absence of randomized studies on anemia in HIV-infected patients with CKD, we report the most up-to-date recommendations for anemia management in CKD214.
1. Characteristics of anemia in CKD: anemia associated with CKD is usually normocytic and normochromic and it is related to a decrease in erythropoietin production by renal peritubular cells, low response of the bone marrow, increased production of hepcidin and decreased availability of iron for erythropoiesis214,215. In HIV-infected patients, anemia has been also associated with treatment with AZT8; however, this drug is not normally part of the current therapeutic regimen.
2. When to begin the anemia study in CKD: when Hb concentration is <11g/dl in premenopausal women and prepubescent patients or <12g/dl in male adults and postmenopausal women.
3. How to study anemia in CKD: to study anemia, it is recommended to carry out the same tests as in the general population: complete blood count, reticulocytes and iron metabolism parameters (iron, ferritin, transferrin and TSI), completing the study with other tests if the clinical circumstances require it (study of hemolysis, vitamin B12 concentrations and folic acid). Intestinal blood loss must be ruled out (when anemia is hypochromic and microcytic or gastrointestinal bleeding is suspected). In patients with stage 5 CKD on hemodialysis (5D CKD), the samples must be obtained immediately before the dialysis session on a midweek day.
4. What are the treatment objectives: treatment of renal anemia should include oral (o.r.) or intravenous (i.v.) iron and erythropoiesis-stimulating agents (ESA) (epoetin alfa and beta, darbepoetin alfa or pegylated epoetin beta), if target Hb concentrations are not achieved with iron alone. In CKD patients, there should be Hb control targets of between 10 and 12g/dl in adults, with symptoms and comorbidities being assessed. If a Hb concentration <10.5-11g/dl is found in patients with stages 3B-5 CKD, they should be referred to the Nephrology department if they are not being followed up, or if they are, the check-up should be brought forward (always after non-renal anemia has been ruled out [due to drugs or gastrointestinal losses, amongst others])215.
5. Iron metabolism required before starting treatment with ESA215: there should be sufficient reserves to achieve and maintain target Hb: TSI ≥20% and <50% and ferritin ≥100ng/ml and <500ng/ml in CKD not on dialysis or <800ng/ml on dialysis. During treatment with ESA, the study of iron metabolism is repeated every three months if patients receive i.v. iron. In patients with ESA without i.v. iron, the test should be carried out monthly until the Hb concentration has stabilised between 10 and 12g/dl. In patients with DM, it is recommended not to begin treatment with ESA if the Hb is not <10g/dl, and if the patient has a history of ischemic stroke, always weighing up the risk/benefit. In patients not treated with ESA, the target is a TSI ≥20% and ferritin ≥100ng/ml. The test must be carried out every 3-6 months.
6. Guidelines for the administration of i.v. iron: it is administered with the aim of preventing deficiency and maintaining iron reserves in order to achieve and maintain the target Hb concentration in patients in whom this is not achieved with o.r. iron. Patients who receive ESA must receive i.v. iron. The doses to be administered are 100-200mg of iron sucrose i.v. or a 500-1000mg infusion of iron dextran or ferric carboxymaltose i.v., repeating as many times as necessary in accordance with the iron parameters (these two types of i.v. iron are those which are currently available in our country). The administration must be carried out in a hospital.
7. Guidelines for the administration of o.r. iron: in adults, at least 200mg of elemental iron must be administered per day. Various existing preparations may be used in accordance with tolerance and price. Pre-dialysis adults, those on home-dialysis and those on peritoneal dialysis who do not achieve adequate iron reserves with o.r. iron, should receive i.v. iron in accordance with the recommendations above. It is considered unlikely that hemodialysis patients will achieve the target with o.r. iron, and as such, they will also require i.v. iron.
8. Indications for treatment with iron (once the other causes of iron deficiency have been ruled out): it is necessary to administer o.r. iron with ferritin <100ng/ml and i.v. iron with ferritin <100ng/ml and intolerance to o.r. iron when Hb is <11g/dl.
9. Indications for treatment with ESA (Epo): if when ferritin has been normalized (≥100ng/ml) the Hb concentration is <11g/dl (or <10g/dl in diabetic patients), it is indicated to assess the introduction of an ESA. If a patient has DM and a history of stroke, the risk-benefit of introducing ESA should be carefully considered. It is advised to individualise the indication and treat promptly until Hb ≥10g/dl has been achieved and then discontinue treatment with ESA and strictly monitor the Hb concentration. The route of administration of ESA is subcutaneous in CKD patients not on dialysis and those on any form of dialysis, with the site of injection always being rotated. In hemodialysis, the i.v. route is normally used, due to greater comfort and to save time. The i.v. route would be indicated in the case of high doses (volume) or recurrent ecchymosis of the injection site. The ESA dose will be that recommended by the Nephrology services. During treatment with ESA, tests will be carried out by measuring the monthly Hb concentration until the Hb target has been achieved or during the dose increases and, once this target has been achieved, the test will be carried out every two months. In dialysis patients, this test will be carried out monthly. The nephrologist will be in charge of adjusting the ESA dose. The ESA dose during intercurrent diseases or surgery must be maintained or increased with the aim of maintaining normal erythropoiesis. The response to ESA is considered to be inadequate when there is an inability to achieve target Hb whenever there are adequate iron reserves, with a dose of ≥30U/kg/week (Epo alfa or beta) or ≥200mg/month (darbepoetin alfa or pegylated epoetin beta) in 4-6 months, or to maintain it at these doses. Transfusions in CKD patients are indicated in those with functional anemia and those who are resistant to ESA with chronic blood loss. The following are the potential adverse effects of treatment with ESA: HBP, seizures, arteriovenous fistula thrombosis and an increase in blood viscosity. Treatment with ESA when Hb is >13g/dl has been related to high rates of CVD, although these rates have not been statistically associated with increased mortality216. The target Hb figure is 10-12g/dl. Anemia tests in CKD patients must include educational programmes for patients, which contain information about this health problem, professional support and lifestyle guidance.
• Section 7.6 recommendations:
1. In HIV-infected patients with CKD, an anemia study will be carried out when hemoglobin concentration is <11g/dl in premenopausal women and prepubescent patients or <12g/dl in adult males and postmenopausal women. (Recommendation based on consensus).
2. Ranges of Hb, ferritin and TSI are the same as in patients without HIV infection. (Recommendation based on consensus).
3. The objective of iron treatment is to achieve a TSI of 20% and a serum ferritin concentration of 100-500ng/ml in pre-dialysis patients or <800ng/ml in dialysis patients. If Hb concentration is lower than 11g/dl (or <10g/dl in diabetic patients), treatment with ESA will be indicated, bearing in mind its risks and benefits. (Quality of the evidence: Moderate Recommendation grade: Weak).
4. Treatment of renal anemia must include o.r. or i.v. iron and ESA if the target Hb concentrations are not achieved with iron alone. (Quality of the evidence: Moderate. Recommendation grade: Weak).
7.7. Management of other chronic kidney disease-related comorbidities in HIV-infected patients
7.7.1. Vaccinations
The response to some vaccines may be suboptimal due to the state of immunosuppression resulting from HIV infection and CKD, which may lead to less development of protective antibodies and a shorter duration of the protection of vaccines. Table 16 displays the vaccines recommended in HIV-infected patients on dialysis.
Dialysis patients have a high risk of hepatitis B and must be vaccinated. The response rate is low, and as such, several doses are required along with regular monitoring of antibody titres. Various studies have highlighted the inadequate response of antibodies against the vaccine: up to half of patients do not develop an anti-HB titre >10UI/L after three subcutaneous doses of 40mg. It is advised to follow the recommendations in the Spanish guidelines on viral infections in dialysis and the consensus document on prophylaxis of opportunistic infections in HIV-infected patients217. Whenever possible, the vaccines should be administered before patients begin the dialysis programme. CKD patients must be vaccinated against hepatitis A and hepatitis B, if they are not immunised, preferably before the start of dialysis.
7.7.2. Non-steroidal anti-inflammatory drugs and analgesics
The use of NSAIDs is associated with three times more risk of developing ARF. Furthermore, they may cause interstitial nephritis and CKD. The decrease in potassium excretion associated with the continuous use of these drugs may favour hyperkalemia from sodium excretion, peripheral edema and HBP and worsen heart failure. Occasional and limited use is well-tolerated if the patient is well-hydrated, do not have concomitant conditions (heart failure, DM or HBP) and there is no major deterioration of renal function. Their extended use is not recommended and renal function must be monitored every 2-4 weeks at the start of treatment. Cyclooxygenase inhibitors have similar effects on the kidney218. Paracetamol does not require dose adjustments. Meperidine, dextropropoxyphene, morphine, tramadol, codeine and their metabolites may affect the central nervous and respiratory systems and they are not recommended in stages 4 or 5 CKD (eGFR <30ml/min/1.73m2). In patients with a decreased GFR, paracetamol or metamizole are advised as the first step. Tramadol is advised as the second step, and if more analgesics are required, fentanyl will be administered, with treatment being introduced in low doses219.
7.7.3. Prevention of contrast-induced nephrotoxicity
HIV-infected patients may at some point during their clinical course receive iodinated i.v. contrast for diagnostic radiological tests. Iodinated contrast may cause direct nephrotoxicity (Table 3), inducing a toxic effect in tubular cells with the possibility of acute tubular necrosis, particularly when it is associated with certain risk situations or potentially nephrotoxic drugs (diuretics, particularly in high doses, aminoglycosides, trimethoprim-sulfamethoxazole, pentamidine, amphotericin B, foscarnet, cidofovir or TDF) or drugs that alter glomerular hemodynamics (NSAIDs, ACE inhibitors or ARBs in situations of salt and water depletion).
In addition to potentially nephrotoxic medication, the factors associated with a greater risk of contrast-induced nephrotoxicity are advanced age, the volume of contrast administered, heart failure, DM, previous renal failure, low output (dehydration, acute myocardial infarction, shock and low blood pressure) and anemia220.
Contrast-induced nephropathy is defined as a deterioration of renal function resulting in a relative creatinine concentration increase of 25% or an absolute creatinine concentration increase of 0.5mg/dl with respect to the baseline value, which occurs during the first three days after contrast administration and is not due to any other mechanism221.
The most important aspect for preventing contrast-induced nephropathy is detecting patients at risk of developing it. In this way, the best treatment is prevention, avoiding the abovementioned risk situations.
The measures for preventing nephrotoxicity include:
• Hydration: fluid therapy is the cornerstone in preventing contrast-induced nephropathy. Many observational and randomized studies have demonstrated its effectiveness. The protocols of fluids used are different, but all studies agree that the combination of i.v. fluid therapy and o.r. hydration is the best regimen. The recommended dose, when logistically possible, is physiological saline solution (0.9%) beginning 12 hours before the procedure at a rate of 1ml/kg/hour, which is maintained until 24 hours after the procedure is finished. Furthermore, o.r. hydration is advised, particularly in outpatients, at least with fluid intake of 500ml on the day the test is carried out and up to 2500ml 24 hours after the test222. If the patient is receiving diuretics, it is recommended that they be discontinued at least 4-5 days before contrast administration. They may be reintroduced 48 hours after contrast administration.
• Drug measures: the only drug that has shown to be effective in the prevention of contrast-induced nephrotoxicity in most clinical trials is N-acetyl cysteine when it is combined with hydration. However, other studies have not found any benefit when combining it with standard hydration223. N-acetyl cysteine is a potent antioxidant that eliminates a wide variety of free radicals, capable of preventing contrast-induced nephropathy, improving renal hemodynamics and preventing the direct renal damage of oxidative stress. The dose to be administered is 1200mg o.r. before the procedure and 1200mg o.r. every 12 hours in the two days following the procedure224.
• Follow-up of patients who have developed contrast-induced nephropathy: the toxic effect of the contrast on the kidneys begins a few minutes after exposure; indeed, the first tubular damage markers appear in urine in the first few hours. However, serum creatinine increases more slowly, beginning on the first day and reaching its peak 3-5 days after contrast administration and it decreases to around its baseline level in 1-3 weeks. The follow-up of these patients will include monitoring of renal function until their baseline creatinine levels have been recovered or renal function has been stable for several weeks in the event of toxicity.
8. COINFECTION WITH THE HEPATITIS C AND B VIRUSES IN CHRONIC KIDNEY DISEASE PATIENTS
8.1. Coinfection with the hepatitis C virus in chronic kidney disease patients
8.1.1. Issues of coinfection with the hepatitis C virus in chronic kidney disease patients
Coinfection with HCV is common in HIV-infected patients. The risk of developing renal disease and its prognosis are worse in coinfected patients. Antiviral therapy for HCV should be determined on a case-by-case basis according to the specific guidelines. In renal diseases that are pathogenically related to HCV (particularly cryoglobulinemic membranoproliferative GN), the treatment of choice is the eradication of HCV. In the event of resistance or intolerance to this treatment, other therapeutic measures (steroids, rituximab, plasmapheresis) have demonstrated their efficacy, at least temporarily. In patients coinfected with HIV-HCV who have advanced CKD or are on chronic dialysis and are in the renal transplantation waiting list (even if the cause of renal damage is not linked to HCV), antiviral treatment is advisable in order to eradicate HCV before transplantation.
Changes in treatment guidelines from the current standard to a regimens based on the new direct-acting antivirals agents, will change the prognosis of these patients225.
It is crucial to address HCV coinfection in the early stages of CKD, and there are several reasons for this strategy. On one hand, the complications associated with HCV-induced end-stage liver disease are one of the main causes of morbidity and mortality in patients with HIV coinfection in the era of ART. This negative influence of HIV infection on the progression of hepatitis C is the main argument for recommending HCV treatment in coinfected patients.
On the other hand, HCV infection in dialysis patients slightly increases mortality and has a negative impact on patient and graft survival after renal transplantation. Consequently, treatment before transplantation may improve the prognosis. However, HCV treatment in patients with renal failure with or without dialysis has some particular characteristics that make treatment more difficult: the use of ribavirin is contraindicated in patients with CrCl below 50ml/min; ribavirin is not eliminated by dialysis, and as such, the increase in its levels in these patients is associated with a very high risk of hemolytic anemia; pegylated IFN must be individualized and adjusted to renal function in order to decrease the risk of toxicity; response rates to treatment are lower than those of patients without renal failure; and, lastly, in patients with transplants, HCV facilitates the onset of some types of GN that may influence graft function and survival and IFN use is contraindicated after renal transplantation due to the risk of graft rejection226,227 and/or acute interstitial nephritis. All of the above reasons suggest the need to plan HCV treatment before transplantation (see section 13.2.3).
Treatment with pegylated IFN and ribavirin in coinfected patients may achieve response rates of 35% in genotype 1 and 73% in genotypes 2 and 3, although some drugs for treating HIV may interfere, causing greater anemia or greater mitochondrial toxicity228.
There are no specific data on hepatitis C treatment in patients coinfected with HIV who also have renal failure. Management of coinfected patients with CKD and/or on dialysis is not well-defined due to a lack of experience and a lack of studies in this regard. HCV treatment of coinfected patients on dialysis is not contraindicated; it must be extrapolated from the data obtained in monoinfected patients and it must be carried out by a multidisciplinary team229,230. For more detailed recommendations on HCV treatment in renal failure patients coinfected with HIV, we recommend consulting the guidelines of the corresponding scientific societies226,227.
8.1.2. Drugs for treating coinfection with the hepatitis C virus
8.1.2.1. Classic drugs
Current treatment of HCV infection is based on the use of one of the two pegylated IFN formulae, peginterferon alfa-2b (Peg-Intron®), or peginterferon alfa-2a (Pegasys®). Both molecules share the same biochemical principle, the binding of a polymer, polyethylene glycol, in order to increase the molecular size, avoid renal clearance and increase the half-life. However, their molecular sizes and pharmacokinetic behaviours are different. Peginterferon alfa-2a is a molecule with a greater number of polymers (40KD), which bind in a branched form. As such, renal clearance of the molecule is very low and the pharmacokinetics in patients with different grades of CKD are modified only with a very low eGFR; in patients with end-stage renal disease on hemodialysis, there is a 25%-45% reduction in clearance and it is recommended to decrease the dose, while in patients with CrCl >20ml/min, the pharmacokinetic parameters are similar to those of individuals with normal renal function231. Exposure of peginterferon alfa-2b increases in patients with renal failure and the doses should be reduced by 50% in cases of severe CKD (CrCl <30ml/min) and 25% in cases of moderate CKD (CrCl <60ml/min)232.
Ribavirin is mainly excreted through the kidneys and in patients with renal failure, episodes of severe hemolysis have been reported. The drug data sheet specifies that it must not be administered to patients with serum creatinine above 2.0mg/dl or CrCl <50ml/min233, although some experts would not exclude any patient, regardless of the CKD stage, from ribavirin treatment234.
8.1.2.2. New direct-acting antivirals
Five drugs have already been approved in Europe for treating adult patients with chronic hepatitis C: telaprevir, boceprevir, simeprevir, sofosbuvir, and daclatasvir, and many molecules are in various stages of clinical development. These new antiviral drugs are expected to dramatically improve care for patients with HCV, including those coinfected with HIV.
In accordance with the data available235,236 most of these new antiviral agents will not require a dose adjustment in patients with mild or moderate renal impairment. The safety and efficacy of most of these new drugs have not been established in patients with severe renal impairment (eGFR <30 mL/min/1.73m2) or end stage renal disease requiring hemodialysis. We strongly advise consulting updated data on dose recommendations for each specific drug.
• Section 8.1 recommendations:
1. In patients who are coinfected with HCV, specific treatment will be indicated in accordance with the therapeutic guidelines. In renal diseases that are pathogenically related to HCV (particularly cryoglobulinemic membranoproliferative GN), the treatment of choice is eradication of HCV with the combinations of antiviral drugs that are currently available. (Quality of the evidence: Moderate. Recommendation grade: Strong).
2. In patients with renal complications due to cryoglobulinemia associated with HCV, in whom antiviral treatment against HCV is ineffective, there is little evidence of favourable effects of treatment with steroids, rituximab or plasmapheresis on renal function. (Quality of the evidence: Very low. Recommendation grade: Weak).
3. Treatment of HCV in patients coinfected with HIV must be carried out before renal transplantation. (Recommendation based on consensus).
4. Given the absence of conclusive studies, HCV treatment regimens for dialysis patients with HIV coinfection will be based on data obtained in patients monoinfected with HCV. (Recommendation based on consensus).
5. IFN use is not advised due to the risk of acute rejection in renal transplantation, except in special circumstances. (Quality of the evidence: High. Recommendation grade: Strong).
6. In patients with an eGFR lower than 50ml/min/1.73m2 ribavirin is contraindicated, although some studies have obtained good results when it is combined with IFN in low doses. (Quality of the evidence: Low. Recommendation grade: Weak).
7. For more detailed recommendations on HCV treatment in patients with renal impairment coinfected with HIV, we recommend consulting the guidelines of the corresponding scientific societies and updated data on the new antiviral drugs against HCV. (Recommendation based on consensus).
8.2. Coinfection with the hepatitis B virus
Coinfection with HBV is much less common than coinfection with HCV. Its prevalence in dialysis patients in Spain is 8.4%10.
In all HIV-infected patients coinfected with HBV (with detectable HBV DNA), ART must include drugs that are active against HBV with the aim of supressing plasma viral load. Some authors believe that active HBV replication despite treatment should be an exclusion criterion for transplantation237.
In these patients, TDF has shown that it is very effective in controlling the replication of the virus and the selection of resistance. The TDF dose is adjusted in accordance with the degree of renal function. Other alternatives such as 3TC, entecavir, adefovir or telbivudine may be used and also require a dose adjustment in accordance with renal function. 3TC and entecavir are active against HIV and HBV and may select resistance mutations in both viruses that may affect the antiviral activity of both drugs. Both TDF and adefovir may cause renal failure and proximal tubulopathy (Fanconi’s syndrome). For more detailed recommendations on management of HBV coinfection we recommend the corresponding clinical guidelines226.
• Section 8.2 recommendations:
1. In all HIV-infected patients coinfected with HBV (with detectable HBV DNA), ART must include drugs that are active against HBV with the aim of making the plasma viral load undetectable. Quality of the evidence: High. Recommendation grade: Strong).
9. USE OF ANTIRETROVIRAL DRUGS IN HIV-INFECTED PATIENTS WITH RENAL impairment OR WHO RECEIVE RENAL REPLACEMENT THERAPY WITH DIALYSIS
Although the cornerstone of HIV infection management is ART, the treatment and prophylaxis of opportunistic infections remains important, and there is an increasing requirement for the use of concomitant treatment for associated comorbidities. Drug use is an issue that is particularly relevant in CKD patients; on one hand, those that are eliminated through the kidneys may undergo an accumulation process and induce systemic toxicity, and on the other hand, there may be decompenzation of previously stable CKD due to direct or indirect toxicity. Some drugs, although may be nephrotoxic both in individuals with normal renal function and in those with CKD, they pose a greater risk of toxicity in patients with impaired function and their use must be avoided insofar as possible. This is the case, for example, with aminoglycosides. Other drugs, although they are not strictly nephrotoxic, may have pharmacological effects that could be harmful in CKD patients. This category includes potassium-sparing diuretics, which may induce hyperkalemia in CKD patients, and NSAIDs, which by inhibiting prostaglandin synthesis reduce renal blood flow and may decrease renal function in these patients238.
As a general rule, in HIV-infected patients with CKD, any drug must be used cautiously, avoiding those which are nephrotoxic and, if a drug is used, it is necessary to know whether it requires a dose adjustment, thoroughly assess the pharmacokinetic profile (bioavailability, route of elimination, metabolism, protein-binding) and analyse the situation of the patient to whom it will be administered (age, sex, weight, height, liver function, renal function, albumin concentration, state of hydration, acid-base balance and other drugs administered). Dose adjustments can be carried out by reducing the dose, extending the administration period, or both.
In dialysis patients, the degree of drug elimination during dialysis must be known and a supplementary dose must be administered after each session if it is eliminated by dialysis. It is important to emphasize all of these aspects, since it has been highlighted that doctors who treat HIV-infected patients often do not make the necessary dose adjustments in individuals with some degree of CKD239.
Tables 17 and 18 display the antiretroviral doses in CKD and dialysis (hemodialysis and peritoneal dialysis). Table 19 displays the dose adjustment for antimicrobials. The drug adjustment tables are based on CrCl estimates using the CG formula. As mentioned i• Section 4.1 this equation cannot be re-expressed for the current methods of measuring creatinine, and as such, it should not be used. On the other hand, CKD-EPI or MDRD-IDMS equations may be used for this purpose, since they are based on standardized procedures for measuring creatinine123-125.
We hope that all drug adjustment tables will soon be expressed with equations that use standardized creatinine (MDRD-IDMS or CKD-EPI), as some guidelines for HIV-infected patients have begun to do126.
9.1. Nucleoside and nucleotide reverse-transcriptase inhibitors
NRTIs are mainly eliminated by the kidneys; in many cases not only by glomerular filtration, but also by tubular secretion. Dose adjustment is particularly important in drugs in which an overdose would lead to an increased risk of renal toxicity, such as with TDF, or those that more frequently cause mitochondrial toxicity, particularly d4T240,241 and ddI242-244. It has been found that patients with renal impairment have an increased risk of developing lactic acidosis245. Another characteristic that is common in NRTIs is their low protein binding and low molecular weight, and as such, they are easily eliminated in dialysis. Therefore, it is recommended to administer the dose after dialysis; in general, it is not necessary to administer extra doses during the dialysis session246. The only drug in this class that is different from the others is abacavir (ABC); it is mainly metabolized by the liver and does not require any adjustment in renal failure, although its administration is recommended after hemodialysis in order to reduce the potential elimination of the drug during the procedure247. In CKD patients who receive 3TC, it is necessary to reduce the dose to 25-50mg/24 hours in patients with advanced renal failure (Table 18), although in certain circumstances, somewhat higher doses may be used (100mg pills) since toxicity has not been observed248-251. In patients with stable CKD, the TDF recommendations established in the data sheet must be followed252-254. It must be borne in mind that the information available on the safety and efficacy of TDF in patients with impaired renal function is very limited. It must only be used in patients with renal failure if it is considered that the benefits outweigh the risks. In general, its use is not recommended in patients with CrCl <50ml/min, except if there are no alternatives. In any case, in patients in whom renal function deterioration is directly related to TDF or is the result of an acute disorder, this drug is not recommended. In addition, strict monitoring of the GFR and of tubular dysfunction markers at the start of treatment and during follow-up is recommended, particularly in patients with a greater risk of developing nephrotoxicity: advanced age, low body weight, a reduced GFR before beginning TDF, concomitant treatment with PIs, comorbidities (DM, HBP, HCV coinfection), nephrotoxic medication and advanced HIV disease (low CD4 levels, AIDS)255.
The possible future availability of the new tenofovir alafenamide fumarate formulation, a prodrug in development that is as effective as the current formulation but with lower renal and bone toxicity256, can be an advance compared to the current formula and can improve the clinical use of this drug.
Emtricitabine (FTC) is also eliminated by glomerular filtration and active tubular secretion in 85%, mostly as an active ingredient without being metabolized257. Its protein binding is very low (<4%) and it requires a dose adjustment in patients with renal impairment. Hemodialysis removes 30% of the dose, and as such, it must be administered after dialysis.
There are currently several fixed-dose NRTI coformulations on the market258. They all contain 3TC or FTC, drugs that require dose adjustments when there is a significant decrease in the GFR. These adjustments are not always in line with those required for the other component in the combination, and as such, the use of coformulations is generally not advised in renal failure patients. Kivexa® (3TC/ABC), Trizivir® (ABC/3TC/AZT) and Combivir® (3TC/AZT) must not be administered to patients with CrCl <50ml/min. In these cases, the drugs must be administered separately and the corresponding adjustments must be made to the doses of each. As mentioned previously, there is little information on the safety of TDF in patients with CrCl <50ml/min, and in general, the use of TDF coformulations with other drugs (for example, Truvada®, Atripla®, Eviplera®) is not advised in patients with CrCl <50ml/min. When it considered essential to use Truvada® (FTC/TDF) and there are no therapeutic alternatives in patients with CrCl <50ml/min, the dosage interval must be increased from once a day to once every 48 hours in individuals with CrCl from 30 to 49ml/min; it must be avoided in patients with CrCl <30ml/min, those in whom TDF-induced toxicity is suspected or those who have unstable renal function258 (Table 18). In terms of coformulations that contain TDF, FTC, elvitegravir (EVG) and COBI (a new CYP3A4 cytochrome inhibitor), as well as what has been mentioned about TDF coformulations with other drugs, we must bear in mind the inhibiting effect of COBI on tubular creatinine secretion, which usually results in an increase in plasma creatinine (<0.3mg/dl in relation to the baseline value) and, therefore, a lower estimated CrCl (~10 ml/min). These changes occur in the first weeks after treatment is introduced, they are not progressive and they are not accompanied by other markers of renal damage, such as proteinuria, normoglycemic glucosuria or urinary sediment abnormalities. There is not yet enough information to determine whether the coadministration of COBI increases the risk of developing tubular toxicity with TDF. At this time, the European Medicines Agency55 data sheet recommends not beginning treatment with this coformulation in patients with CrCl <90ml/min, unless it is considered that this is the preferred regimen for the individual patient. The increase in creatinine (>0.3mg/dl from the baseline value) or the decrease in CrCl (to less than 70ml/min), moderate or intense hypophosphatemia (<2mg/dl) and urine abnormalities (glucosuria with normoglycemia, proteinuria) are the main findings that must make us suspect renal toxicity. In these cases, the study must be extended, with more specific tests being carried out, amongst them FEP and urate, APR in urine, serum and urinary potassium and the acid-base balance in blood. It is recommended to discontinue TFV/FTC/EVG/COBI if CrCl decreases below 70ml/min during treatment, unless the potential benefit of this combination in individual patients outweighs the potential risks of continuing with treatment. In any case, it must always be discontinued if CrCl decreases below 50ml/min, since it is necessary to adjust the dosage interval for TDF and FTC and this cannot be carried out with the fixed-dose combination pill258 (Table 18).
9.2. Non-nucleoside reverse-transcriptase inhibitors
NNRTIs are metabolized by the liver and the renal elimination of the active ingredient is minimal. NVP protein binding is 60%; this percentage is higher for delavirdine and EFV, in which it is 98% and 99% respectively259. NVP, with <3% urinary excretion of the active ingredient, does not require a dose adjustment in CKD patients. In hemodialysis patients, there may be certain elimination of the drug during the procedure, and as such, it is recommended that on the day of dialysis, the corresponding NVP dose be administered after dialysis has finished260,261. NVP is also cleared though peritoneal dialysis, although it does not seem to have any effect on plasma concentrations262,263 (Table 17).
The pharmacokinetic data on EFV in CKD patients who are not on treatment with dialysis are limited, but it is assumed that they do not require a dose adjustment, since less than 1% is eliminated as an active ingredient in urine. EFV clearance, both by hemodialysis and peritoneal dialysis is low, and as such, it is not necessary to make dose adjustments or modifications at the time of administration264,265. Etravirine (ETR) is mainly excreted through the liver and less than 1.2% is eliminated by the kidneys; as such, it is not expected that a dose adjustment will be required as GFR decreases. Nor is it expected that will be eliminated by hemodialysis or peritoneal dialysis, given its high protein binding266. In addition to ETR, another new drug in this family, RPV, is available. Its metabolism and elimination are mainly carried out by the liver (CYP 450) and the gastrointestinal tract (85% in faeces) and to a lesser extent by the kidneys (<6%); dose adjustments are not necessary in mild or moderate renal failure. It is recommended to monitor it closely in patients with more advanced CKD, due to the potential increase in plasma concentrations. It is unlikely that it will be eliminated during hemodialysis or peritoneal dialysis due to its strong protein binding267-270. It should be borne in mind that RPV may have a weak inhibiting effect on tubular creatinine secretion, which may cause changes in plasma creatinine and in the eGFR. RPV has also been marketed in a coformulation with FTC and TDF (Eviplera®). As with the other coformulations that contain TDF, Eviplera® use is not recommended in patients with CrCl <50ml/min, since the required dose adjustments cannot be made.
9.3. Protease inhibitors
PIs are mainly metabolized in the liver. Urinary excretion is around 10% of the active ingredient for IDV and 7.5% or less for other drugs in this family. Most PIs have wide distribution volumes and to a great extent bind to plasma proteins, IDV approximately 60% and other drugs in this class 90% to over 98%271. No PI currently available requires a dose adjustment in CKD patients. Although a limited number of patients have been studied, the great affinity that PIs have for plasma proteins prevents their clearance by hemodialysis261-263,271-273, with the exception of NFV, for which there are contradictory data274,275 (Table 17) and LPV/r, which showed levels 44% lower than expected276. ATV and DRV behave like the other drugs in this family and there are no special dose or regimen adjustment recommendations in the different degrees of CKD or in renal replacement therapy (Table 17)277,278.
9.4. Fusion inhibitors
Enfuvirtide, the only fusion inhibitor currently available, has strong protein binding (≈92%). This drug is a peptide that is partly converted to an inactive deaminated metabolite and which along with the active ingredient undergoes metabolism to amino acid residues279. In studies carried out in patients with CKD, it has been observed that clearance is not altered and that it is not necessary to adjust the dose. Furthermore, it is unlikely that it will be cleared in dialysis280,281 (Table 17).
9.5. Integrase inhibitors
There are currently three INSTI: RAL, EVG and DTG. EVG has only been approved by the regulating agencies in coformulation with TDF/FTC/COBI282-287.
In healthy volunteers in whom the absorption, metabolism and excretion of RAL was studied, it was observed that 32% of the drug was eliminated through the kidneys, 9% as an active drug and 23% as a glucuronidated metabolite. Furthermore, 51% was eliminated in faeces as an active ingredient without being metabolized and the main route of metabolization identified was through the liver by glucuronidation by UDP-glucuronyltransferase; therefore, few interactions are expected with the drugs metabolized through the cytochrome P450 (CYP) system288. A study of RAL has been carried out in CKD patients without any clinically significant differences being found in the pharmacokinetic parameters between CKD patients and healthy subjects289. It is therefore not necessary to carry out any dose adjustment. Due to not knowing to what extent it is dialysed, it is recommended to administer the dose after dialysis (Table 17)288,289. EVG is mainly metabolized by the cytochrome P450 route, particularly by CYP3A4. Glucuronidation is only a minor metabolic route. Therefore, the minimum and steady state concentrations of EVG substantially increase when RTV or COBI are administered. The half-life in plasma also increases, which allows it to be administered once a day290. In patients with severe CKD (CrCl <30ml/min) a pharmacokinetics study of EVG boosted with COBI was carried out, without differences being found when compared with healthy subjects282,283. There is still no information about the pharmacokinetics of EVG in dialysis patients, although due to the fact that it is mainly metabolized in the liver and there is little renal elimination of the active drug, it is not expected that major dose adjustments will be necessary. As mentioned above, the EMA recommends not to initiate ART with the coformulation of TDF,FTC, EVG and COBI in patients with CrCl<90 ml/min. The inhibiting effect of COBI on the tubular secretion of creatinine must be taken into account, since it may result in a slight increase in plasma creatinine and, therefore, a decrease in estimated CrCl (~10ml/min). It is recommended to discontinue the drug if CrCl decreases below 70ml/min282,283.
DTG is mainly metabolized by the liver through glucuronidation by UGT1A1, while CYP3A is a minority alternative route. Renal excretion is <1%, and no dosage adjustment is required in patients with mild, moderate or severe (CrCl <30 mL/min, not on dialysis) renal impairment284. As with COBI and RPV, DTG is also an inhibitor of tubular creatinine secretion, which is demonstrated by a slight change in serum creatinine concentration and estimated CrCl (~10 ml/min) from the start of treatment, which does not result in renal dysfunction. The absence of an effect of this increase in creatinine on renal function was well documented in a placebo-controlled clinical trial in healthy volunteers, in which DTG administration reduced CrCl by 10%-14%, but did not have any effect on the GFR measured by renal clearance of iohexol or on renal plasma flow51.
9.6. CCR5 co-receptor antagonists
Maraviroc (MVC) is mainly metabolized by the liver by CYP3A4. It is mainly eliminated by the gastrointestinal route (76%) and only 25% is eliminated in urine. 33% of the eliminated drug is active ingredient that has not been metabolized. Although there are no data on the behaviour of the drug in patients with different degrees of CKD, simulation studies have been carried out and a dose adjustment is currently only recommended in patientswith renal impairment who are receiving potent CYP3A4 inhibitors, such as PIs (except tipranavir/RTV), ketoconazole, itraconazole, clarithromycin, and telithromycin. In the presence of these metabolic inhibitors, renal clearance may reach 70% of total MVC clearance and, therefore, in this case, there may be a significant increase in exposure to MVC291 (Table 17) and its side effects, among others, dizziness and postural hypotension, and therefore, its use is only recommended in the event that the benefits outweigh the risks and strict monitoring is observed.
10. REGIMENS FOR THE ANTIRETROVIRAL THERAPY OF CHOICE AND ITS DRUG DOSE ADJUSTMENT IN CHRONIC KIDNEY DISEASE
There is little clinical evidence about what should be the regimen of choice and its adequate dosage in CKD patients7,8,28,252,253,264,274,276,281,292-296. Nevertheless, a series of general recommendations are summarized in Table 20.
NRTIs are eliminated through the kidney, and it is therefore necessary to reduce the dose in patients with impaired renal function. An overdose or underdose may lead to toxicity or virologic failure respectively297. An exception is ABC, whose urinary excretion is low, and as such, a dose adjustment is not necessary (see the data sheet).
NNRTIs, PIs, INSTI and fusion inhibitors do not require a dose adjustment in patients with renal function deterioration7,8,28,252,253,264,274,276,281,292-296. In these patients, fixed-dose drug combinations must be avoided due to drug dosage difficulties.
A study that analyzed patients who received different PIs showed that patients treated with ATV and DRV have high urinary concentrations of the drugs, and crystalluria composed of these drugs (8.9% and 7.8% respectively) was detected, contrarily to LPV, which had lower concentrations of the drug in urine and an absence of crystalluria49.
• Section 10 recommendations:
1. All fixed-dose antiretroviral drug combinations are contraindicated (Atripla®, Eviplera®, Stribild® and Triumeq®) due to dosage difficulties of NRTIs in patients with a GFR <50ml/min. (Quality of the evidence: High. Recommendation grade: Strong).
2. A HLA B57-01 test must be carried out in all patients who are expected to receive ABC, in order to avoid the risk of hypersensitivity to the drug. (Quality of the evidence: High. Recommendation grade: Strong).
3. ART in CKD patients must follow the same recommendations as in patients without renal involvement, although it is not advised to administer the TDF/FTC/COBI/EVG coformulation (Stribild®) in patients with a GFR <70ml/min. If there are no contraindications, the combination of ABC (or TDF adjusted to the GFR as an alternative) plus 3TC (adjusted to the GFR) with an NNRTI, a PI boosted with RTV an INSTI other than EVG may be used. (Quality of the evidence: Moderate. Recommendation grade: Strong).
4. If TDF or ABC cannot be administered, the combination of PI boosted with RTV and 3TC (adjusted to the GFR) or RAL may be used, or in very selective cases, it may be simplified to a monotherapy: PI boosted with RTV. (Quality of the evidence: Low. Recommendation grade: Strong).
11. MANAGEMENT OF DIALYSIS PATIENTS
11.1. Prevalence and prognosis of HIV-infected patients on dialysis
The prevalence of HIV infection in patients who receive renal replacement therapy with dialysis varies widely depending on the geographic region. Studies carried out in the European Union indicate that the prevalence of HIV infection in CKD and dialysis patients is low, at around 0.5%10-12. In Spain, the prevalence recorded in the latest Spanish Registry of Renal Diseases report of 2007 is 0.3%298.
In the pre-ART era, prognosis in dialysis was very poor, and it was even questioned whether renal replacement therapy should be introduced in these patients. During the 1990s, a very significant improvement was observed and recent descriptive analyses in the United States and Europe have highlighted mortality rates that are very close to those of the general population with CKD on dialysis212,299. This positive development has likewise allowed access to renal transplantation with very satisfactory short- and medium-term results, similar to those of other risk groups300-304.
Due to this improved survival, and although the rate of incident patients with end-stage renal disease seems to have reached a plateau, the prevalence observed in the United States and in some European countries has slightly increased in recent years305,306. In Europe, a recent survey carried out in 2008 in EuroSIDA study centres showed differences between countries with a mean prevalence of 0.46%11. In Spain, the most recent data, recorded in the annual reports of national and autonomous registries of renal patients and in the survey carried out by the HIV Infection in Dialysis Study Group (GESIDA study 48/05), currently show very stable prevalence values (0,4 %, 0.5 %, 0.3 %, 0.5 % in 2005, 2006, 2007 and 2011 respectively)10,298,307.
The control of HIV infection with new antiretroviral drugs is the determining factor in this change. Several studies have demonstrated greater survival in patients who receive ART and a higher CD4 lymphocyte count. However, the continuous introduction of new antiretroviral drugs, the need to adjust the dose of some drugs and the frequent use of concomitant treatments that may interfere with the metabolism of antiretrovirals and vice-versa28,212,305,306 make management of HIV-infected patients who require renal replacement therapy particularly complicated. A multidisciplinary approach, with good communication between the HIV infection specialist and the nephrologist, and revision and consensus documents, such as ours, may be decisive in improving clinical results in these individuals.
11.2. Dialysis method of choice and vascular access in hemodialysis
The most used renal replacement therapy method in HIV-infected patients in Spain is hemodialysis (74%) versus peritoneal dialysis (26%)10. The prevalence of HIV-infected patients on dialysis in Europe is also low and hemodialysis is also the most used method11. In Spain, the greatest source of contagion with the HIV infection in these patients was the parenteral route (due to former drug use by this route), with there therefore being a high percentage of patients coinfected with HCV10. The prevalence of HBV coinfection is much lower in these individuals11.
Few studies have analyzed survival of HIV-infected patients on dialysis; in general, survival is similar in both dialysis methods308. Only one cohort study carried out in Spain reported higher mortality in patients on peritoneal dialysis than in those on hemodialysis309.
In relation to potential contagion with the virus in hemodialysis units, strict compliance with precautions of universal control of infections is sufficient for preventing the transmission of the HIV infection in dialysis. There is no evidence that contraindicates these patients being dialysed in a general dialysis unit with implementation of the universal precautions recommended in the S.E.N. guidelines310. In the case of coinfection with HBV, the patient must receive dialysis in a special HBV unit. In the case of HCV, the measures described in the S.E.N. clinical guidelines will be maintained. Studies in the pre-ART era identified the virus in peritoneal effluent, and as such, the latter should be handled as a contaminated body fluid. Patients must be informed and trained in the handling of contaminating material. The risk of accidental transmission due to percutaneous exposure (0.3%) or via mucous (0.09%) is very low, but it may occur, and as such, in the event of an accident, prophylactic measures must be implemented as soon as possible after exposure28,212,213.
The dialysis method of choice in HIV-infected patients should be chosen in accordance with the same recommendations as for the population without HIV infection. The patient may choose the method once they have been informed about the advantages and disadvantages of each of them, provided that the Nephrology specialist does not consider that one method may be more beneficial or, on the contrary, contraindicated, as the case may be.
With regard to vascular access in hemodialysis patients, the first option should be an arteriovenous fistula whenever possible and tunnelled catheters should be avoided. In peritoneal dialysis, the catheter will be inserted using the universal precautions.
In ARF, in which dialysis is expected to be temporary, it is recommended that a temporary catheter be inserted at the jugular or femoral vein according to the patient’s characteristics. If ARF is considered to be irreversible and results in end-stage renal disease, as mentioned above, definitive vascular access will be considered if the patient prefers hemodialysis, or a peritoneal catheter, if they opt for peritoneal dialysis. If it is considered that the patient may be a candidate for chronic dialysis, the insertion of a tunnelled catheter may be considered at first in order to avoid risk of contagion with successive catheter exchanges. HIV-infected patients may receive dialysis in an acute admissions unit along with other patients, provided that there is no HBV coinfection, in which case they would require a special unit. In the case of accidental puncture, the measures indicated in the S.E.N. guidelines for prevention of transmission will be followed310.
In the case of an HIV-infected patient with CKD that is already known who will be included in a chronic hemodialysis programme, it is recommended to use an autologous arteriovenous fistula as the vascular access of choice, followed by prosthesis (polytetrafluoroethylene graft) and, lastly, a tunnelled catheter.
• Section 11.1 and 11.2 recommendations:
1. Treatment with dialysis cannot be contraindicated due to HIV infection in any patient. (Recommendation based on consensus).
2. In HIV-infected patients on dialysis, the universal prevention and disinfection measures must be strictly adhered to both in hemodialysis and in peritoneal dialysis, as with patients not infected with HIV. (Quality of the evidence: Moderate. Recommendation grade: Strong).
3. There are no contraindications with regard to these patients receiving dialysis in a general dialysis unit with the universal precautions recommended in the guidelines. (Quality of the evidence: Moderate. Recommendation grade: Weak).
4. There is no evidence for isolation in different rooms in the case of coinfection with HCV and HIV. (Quality of the evidence: Moderate. Recommendation grade: Weak).
5. In the case of coinfection with HBV, the patient should receive dialysis in a special HBV unit. (Quality of the evidence: High. Recommendation grade: Strong).
6. The method of dialysis in HIV-infected patients will be selected in line with the same recommendations as for the population without HIV infection. Patients will decide the method once they have been informed about the advantages and disadvantages of each one, provided that the nephrologist does not consider that a particular method is contraindicated. (Recommendation based on consensus).
7. If the treatment chosen is hemodialysis, the first vascular access option must be arteriovenous fistula, the second option is polytetrafluoroethylene prosthesis and lastly, the tunnelled catheter. In peritoneal dialysis, the catheter will be inserted taking the universal precautions. (Recommendation based on consensus).
8. In the case of peritoneal dialysis, it is suitable for the patient to carry it out and handle all the material themselves at home. It is recommended that after eliminating the dialysate in the lavatory, a disinfectant such as bleach be added, waiting 30 minutes before flushing to the general network. Likewise, the lines and bags of peritoneal fluid should be deposited in bins for contaminating material after use, which the patient may bring to the health centre for elimination. (Recommendation based on consensus).
9. If there is accidental percutaneous exposure or exposure through mucous, prophylactic treatment will be introduced as soon as possible following exposure in accordance with the indications of the corresponding specialist. (Recommendation based on consensus).
10. Patients with CKD and HIV infection must be vaccinated against hepatitis A and B if they are not immunised, preferably before the start of dialysis. (Recommendation based on consensus).
11.3. Regimens for the antiretroviral therapy of choice and its dose adjustment in dialysis
The ART regimens of choice in dialysis patients are the same as for CKD patients (see section 10), but some considerations should be borne in mind with regard to administration of the drugs in hemodialysis or peritoneal dialysis patients and their adjustment to renal function (Tables 17 and 18). It is important to note that the information on ART dosage and administration in peritoneal dialysis patients is very poor297. NRTIs are eliminated through the kidneys and are therefore eliminated by dialysis, and as such, they must be administered after dialysis. As previously mentioned, NNRTIs do not require a dose adjustment, but in the specific case of NVP, an additional 200mg dose is indicated after each hemodialysis session. There is also a particular recommendation in the case of PIs: ATV administration must be boosted with RTV in dialysis patients, since they have low ATV concentrations.
The ideal ART regimen in naïve HIV-infected patients on dialysis must include ABC or TDF with 3TC/FTC, in combination with a third drug, which may be EFV, a PI boosted with RTV or RAL. In patients with effective ART but with the adverse effects of NRTIs, the treatment regimen may be simplified to monotherapy with LPV/r or DRV/ritonavir311,312 or a combination of RAL with a PI boosted with RTV. In patients with virologic failure, the rescue therapy must be based on the results of the resistance test. In patients who are on the waiting list and are close to receiving a renal transplant, with the aim of avoiding drug interactions with the immunosuppressants and renal toxicity of the graft, the combination of choice would be ABC with 3TC and RAL (or EFV as an alternative)313.
• Section 11.3 recommendations:
1. The same recommendations as in the previous section (on the use of antiretroviral drugs in HIV-infected patients with renal failure).
2. In patients who are on the waiting list and are soon to receive a renal transplant, with the aim of avoiding drug interactions with immunosuppressants and renal graft toxicity, the combinations of choice would be ABC with 3TC (adjusted to the GFR) and RAL/DTG (or EFV as an alternative). (Quality of the evidence: Low. Recommendation grade: Strong).
12. CRITERIA FOR INCLUDING HIV-INFECTED PATIENTS IN RENAL TRANSPLANTATION WAITING LIST
12.1. Criteria for including HIV-infected patients without hepatitis C or B virus coinfection in renal transplantation waiting list
The criteria for renal transplantation in HIV-infected patients are the same as in the general population, but they must also meet criteria related to HIV infection. HIV-infected patients on dialysis or in pre-dialysis must not be excluded a priori from receiving a renal transplant. It is recommended that they be referred to a renal transplantation unit with experience in these types of transplantations before considering not to include them. The criteria for inclusion of HIV-infected patients on the renal transplantation waiting list are summarized in Table 21.
In our country, criteria are based on the recommendations of GESIDA/Transplant Infection Study Group [GESITRA]/National Transplant Organization and are very similar to those used by other scientific societies. The specific criteria related to HIV-infection have been defined in Western countries and are based on the opinion of experts. They are as follows237,314-316:
1. Clinical criteria: ideally, patients should not have had any AIDS-defining event. Nonetheless, some opportunistic infections that are potentially preventable or treatable (tuberculosis, esophageal candidiasis and pneumonia due to Pneumocystis jiroveci) are not yet considered to be exclusion criteria in most Western countries. These patients may have to undergo a clinical evaluation in order that each case may be analyzed individually before rejecting them from the renal transplantation waiting list300.
In the case of tumours, if there is a history of cutaneous Kaposi’s sarcoma, there should be a five year disease-free period before transplantation. For in situ anogenital carcinoma, squamous and baseline cell skin carcinoma and solid tumours with curative treatment, the patient must be disease-free for five years prior to transplantation. However, this period may be individualized according to the stage of the tumour. More recently, researchers in the United States have suggested extending this criteria to all AIDS-defining events, with some exceptions: progressive multifocal leukoencephalopathy, chronic cryptosporidiosis, lymphoma and visceral Kaposi’s sarcoma300.
2. Immunological criteria: the CD4+ lymphocyte count must be greater than 200 cells/ml.
3. Virological criteria: the viral load (HIV RNA) must be undetectable in plasma using ultrasensitive techniques (<50 copies/ml) before transplantation and patients should have the opportunity to receive an ART regimen that is effective in the long-term in the post-transplantation period. In patients without pre-transplantation ART criteria, low levels of viremia may be permitted, but ART must be introduced after transplantation.
4. Other criteria: all patients must have a positive psychiatric evaluation. Patients with active recreational drug use will be excluded. A drug-free period of two years is recommended for heroin and cocaine and six months for other drugs, including alcohol. Patients who are stable on a methadone programme should not be excluded. Lastly, patients must have an adequate degree of social stability.
• Section 12.1 recommendations:
1. HIV-infected patients with CKD on dialysis or in pre-dialysis (GFR <20ml/min/1.73m2) who meet the general criteria after renal transplantation evaluation and the specific HIV infection criteria must be included on the renal transplantation waiting list. (Quality of the evidence: High. Recommendation grade: Strong).
12.2. Criteria for including HIV-infected patients with hepatitis C or B vírus coinfection in renal transplantation waiting list. (See section 8)
HBV and, in particular, HCV coinfections are very prevalent, since they share transmission routes with the HIV. Management of HBV virus infection is currently not a problem, given that there are different drugs (3TC, TDF, entecavir) effective to control viral replication.
HCV infection is a major problem, given the high prevalence in our setting (30%-40%). In renal transplantation, chronic HCV infection is associated with a greater risk of infections and is a risk factor for mortality and graft loss. Likewise, immunosuppressant treatment may reactivate the HCV infection and antiviral treatment with IFN is not advised during transplantation due to the high risk of triggering acute rejection with renal graft loss. HCV infection also behaves more aggressively in HIV-infected patients, which may be an additional risk in coinfected patients who receive transplants. In a US multicentre study, these patients had a greater rate of severe infections with a tendency for worse survival302. In a Spanish study, recipients coinfected with HIV-HCV had a worse renal graft outcome303.
A complete study on HIV-HCV patients on dialysis is currently advised, which includes carrying out a gastroscopy and a hepatic hemodynamic study with a transjugular liver biopsy. Before transplantation, treatment with IFN or IFN-ribavirin must be considered. If HCV-RNA negativization is achieved, a renal transplant is indicated. If a positive HCV viral load persists, transplantation may be carried out in the absence of advanced liver disease.
The role of new PIs has not currently been established in the treatment of HCV infection in HIV-infected patients on dialysis or renal transplant recipients.
• Section 12.2 recommendations:
1. In patients who meet the criteria of the previous section (with regard to recommendations on the criteria for inclusion on the renal transplantation waiting list in HCV or HBV coinfected patients) and who are coinfected with HCV and/or HBV, a complete evaluation of their liver disease (viral load, an ultrasound, a hepatic hemodynamic study and a transjugular liver biopsy) must be carried out. (Quality of the evidence: High. Recommendation grade: Strong).
2. In patients with HIV and HCV coinfection who are candidates for a renal transplant on its own, antiretroviral treatment must be assessed before performing transplantation. (Quality of the evidence: High. Recommendation grade: Strong).
3. Patients who are coinfected with HIV and HBV who are candidates for a renal transplant on its own must receive antiviral treatment before and after transplantation. (Quality of the evidence: High. Recommendation grade: Strong).
4. If the patient has advanced chronic liver disease, renal transplantation on its own is not advised and the possibility of combined liver and renal transplantation (LRT) will be evaluated, although the experience is very limited and its indication should be individualized in accordance with indications in point 9.3. (Quality of the evidence: Moderate. Recommendation grade: Weak).
12.3. Criteria for including HIV- infected patients with end-stage liver disease in combined liver and renal transplantation waiting list.
The criteria that indicate a combined LRT are being discussed in patients who are not infected with HIV. It is well agreed that advanced liver disease is a contraindication for an isolated kidney transplantation and, vice versa, chronic renal failure is a contraindication for an isolated liver transplantation. The problem is that in combined LRT, on some occasions, the liver is transplanted prematurely in patients with end-stage renal disease, and on other occasions, the kidney is transplanted in patients with early stages of renal failure.
Renal failure previous to orthotopic liver transplantation (OLT) is an independent risk factor for mortality, and as such, since the MELD model was adopted in 2002, the indication of combined LRT has increased greatly. This improves the survival of patients with the indication of liver transplantation when they are on dialysis or have advanced renal failure. However, it has not been demonstrated that this model benefits survival with respect to isolated OLT in patients with mild-moderate renal failure317-321.
In hepatorenal syndrome, it is very important to establish the potential reversibility of ARF in order to be able to indicate a combined LRT or an isolated OLT. Some data indicate that LRT only has survival benefits over OLT in patients with hepatorenal syndrome that requires dialysis over a minimum of 6-8 weeks after transplantation. Of the patients who received OLT, 89% recovered renal function without the need for dialysis beyond the month following OLT and mean serum creatinine 265 days later had decreased to 1.55mg/dl, which confirms the reversibility of renal function in patients with hepatorenal syndrome after OLT; and only 3 of the 80 patients required chronic hemodialysis (3.5%)322.
Renal biopsy is also very useful for establishing the irreversibility of renal failure and for indicating combined LRT or OLT on its own. The degree of interstitial fibrosis in renal biopsies is a marker of renal progression to end-stage renal disease, and as such, it has been suggested that when it is more than 30%, the indication for a LRT must be considered. Glomerulosclerosis is also a marker of progression to end-stage CKD, although not as strong as the previous marker. According to Easen et al., patients with an eGFR <30ml/min/1.73m2 should have a renal biopsy. However, it is not always possible to perform a biopsy given the risk of complications due to bleeding in this type of patient320.
As a result of these doubts, in 2008, experts of the American Society of Transplant Surgeons, the American Society of Transplantation, the United Network for Organ Sharing and the American Society of Nephrology developed a consensus document on indications for LRT320. The following indications were established:
1. End-stage liver disease (ESLD) and CKD on dialysis.
2. ESLD and ARF on dialysis for a minimum of eight weeks.
3. ESLD CKD with a GFR <30ml/min/1.73m2.
4. In ESLD and ARF of unknown origin with a GFR <30ml/min/1.73m2 and fibrosis and/or glomerulosclerosis >30% in the biopsy, a LRT is also recommended320.
In an effort to avoid an unnecessary renal transplantation in patients with ESLD, the International Liver Transplantation Society subsequently also included combined LRT in patients with ESLD and CKD with a GFR <30ml/min/1.73m2 and proteinuria >3g/day and in patients with ESLD and ARF who require dialysis over more than six weeks321,323.
The current recommendations for indicating combined LRT in patients with ESLD and renal failure are detailed in Table 22. These recommendations are based on populations without HIV infection. The experience of LRT in HIV-infected patients is very limited.
• Section 12.3 recommendations:
1. For combined LRT, it is necessary to assess HIV-infected patients with CKD and chronic liver disease who meet the general renal transplantation criteria, as well as criteria specific to HIV infection and those specific to LRT. (Quality of the evidence: Low. (Recommendation based on consensus).
2. The experience of combined LRT in patients with HIV infection is very limited, and as such its indication must be individualized. (Quality of the evidence: Low. Recommendation based on consensus).
12.4. Criteria for including HIV- infected patients with diabetes mellitus in combined renal and pancreas transplantation waiting list
Combined renal and pancreas transplantation (RPT) is indicated in patients with type 1 DM and CKD on dialysis or in predialysis younger than 50 years of age and without severe vascular disease. Pancreas transplantation may be carried out simultaneously or subsequently to renal transplantation324,325. The results of RPT have been improving, with renal graft survival rates being comparable to those of renal transplants in patients without DM (85% and 68% after 5 years and 10 years respectively). Pancreas survival is better in simultaneous transplantation and very significant figures have also been achieved (73% and 55% after 5 and 10 years, respectively)324-327. The main risk of RPT is surgical complications (thrombosis, fistulae, pancreatitis), infections and a higher risk of pancreatic and renal rejection. Its main advantages are remission of diabetic polyneuropathy, better metabolic control, stability of the retinopathy and avoiding the recurrence of diabetic nephropathy in transplanted kidneys. The change in established macroangiopathy is small326,327. The experience of RPT in HIV-infected patients is limited (see section 12.4) and the criteria for inclusion on the waiting list are displayed in Table 23.
• Section 12.4 recommendations:
1. For RPT, all patients with HIV infection, type 1 DM and advanced CKD who meet the general renal transplant criteria, specific HIV infection criteria and RPT criteria must be assessed. (Quality of the evidence: Low. (Recommendation based on consensus).
2. RPT experience in HIV-infected patients is very limited and its indication must be individualized. (Quality of the evidence: Low. (Recommendation based on consensus).
13. MANAGEMENT OF RENAL TRANSPLANT RECIPIENTS
Until a few years ago, HIV infection was an absolute contraindication for any type of transplantation. The fear that the immunosuppression necessary in the post-transplant period could accelerate progression to AIDS and development of opportunistic infection, in addition to the poor prognosis of HIV infection, resulted in a high degree of rejection of this method of therapy. Nevertheless, in the few cases in which, for whatever reason, renal transplantations were carried out in the pre-ART era, the results were not at all poor328,329. The widespread use of ART since 1996 resulted in a radical change in the prognosis of HIV infection and it has become a manageable and controllable chronic disease.
13.1. Survival of HIV-infected renal transplant recipients
In the last ten years, renal transplantation experience has notably increased in HIV-infected patients. Initially, there was experience with isolated cases or small retrospective case series. Subsequently, prospective studies were published that included a greater number of renal transplantation cases in HIV-infected patients. The most important study published to date is that of Stock et al., which included 150 renal transplant recipients, carried out between 2003 and 2009 in 19 transplantation centres in the United States302. Patient and graft survival was 95% and 90% after one year and 88% and 74% after three years respectively. The survival results were considered to be intermediate between those obtained in the general transplanted population of the United States and those of a high-risk renal transplantation group. In a recent study that included 36 HIV-infected patients who received a renal transplant in 13 Spanish hospitals, the results are very similar, with graft survival of 91.6% and 86.2% one and three years after transplantation330.
We can draw the following conclusions from experience in the ART era and from modern immunosuppressant treatments:
Medium-term survival (of patients and grafts) in adequately selected HIV-infected patients may be similar to medium-term survival in patients without HIV infection.
There is evidence that in the period following transplantation patients may have good virological and immunological control using ART.
It has not been demonstrated that immunosuppressant treatment in the post-transplantation period leads to a poor outcome of the HIV infection or that there is greater progression to AIDS or a higher number of opportunistic infections or tumours related to AIDS11,313.
Table 24 summarizes the main survival data in the United States before and during the ART era302,331.
• Section 13.1 recommendations:
1. Renal transplantation should be considered as a valid treatment in adequately selected HIV-infected patients, since patient and graft survival is similar to that of patients without HIV infection and there is no evidence of a poor outcome of the HIV infection in the post-transplantation period. (Quality of the evidence: High. Recommendation grade: Strong).
2. It is recommended to create multidisciplinary teams in renal transplantation and HIV-infectious diseases for the clinical follow-up of these patients. These teams will together analyze the potential pharmacological interactions of antiretroviral or immunosuppressant drug dose changes, or any introduction or discontinuation of any of these drugs. (Recommendation based on consensus).
13.2. Specific considerations about renal transplantation in HIV-infected patients
The complexity of patients with HIV who undergo renal transplantation requires the multidisciplinary collaboration of nephrologists, infectious disease specialists and transplantation experts, as well as other support specialists (chemists, psychologists, psychiatrists, social workers, etc.). It also involves a series of particular characteristics, both in the pre-transplantation and post-transplantation periods, essencial to be evaluated in order to obtain the best results. In any case, any patient infected with HIV virus who may benefit from renal transplantation must be considered for inclusion on the waiting list provided that they meet the criteria displayed in Table 21. In this regard, it should be highlighted that, in one study carried out in the United States, only 20% of the theoretical HIV (+) transplantation candidates had been included on the waiting list, compared with 70% HIV (–) patients over the same period of time332. In Spain, a GESIDA/S.E.N. study of cases and controls demonstrated that, while 62% of the 66 HIV negative patients on dialysis had been placed on the renal transplantation waiting list, only 17% of the 66 HIV-infected patients had been included108.
13.2.1. Pre-transplant period
13.2.1.1.Donor source for kidney transplantation in HIV-infected patients
HIV-infected patients on the transplantation waiting list may receive a renal graft from a cadaveric donor or a living donor who is seronegative for HIV. In previous studies, graft loss frequency was considerably lower when the organ was from a living donor302,333.
The use of organs from HIV-infected donors is completely contraindicated in our setting. Recently, limited experience with HIV-infected recipients who received a renal graft from HIV-infected donors was published in South Africa with good results. This situation must be set within the context of countries with significant economic, health and transplant access limitations.
There is not sufficient information on safety and long-term efficacy, and as such, donor kidneys from HIV-infected patients currently must not be accepted334,335.
• Section 13.2.1.1 recommendations:
1. Kidney donor selection criteria for HIV-infected patients are similar to those of the general population (cadaveric donor or living donor). (Quality of the evidence: Moderate. Recommendation grade: Strong).
2. The use of renal grafts from HIV-infected donors is contraindicated. (Quality of the evidence: Low. (Recommendation based on consensus).
13.2.1.2. Antiretroviral therapies of choice in renal transplant recipients
It is important to adjust the type and dose of antiretroviral drug to the patient’s advanced renal failure situation. Whenever possible, the administration of antiretroviral drugs with mitochondrial toxicity should be avoided (for example ddI, d4T). The dose adjustment requirements in advanced renal failure and dialysis may be consulted in this document, in the GESIDA/PNS ART document, which is regularly updated (www.gesida.seimc.org) and at other accredited sources (http://www.interaccioneshiv.com/, http://www.hiv-druginteractions.org; http://www.interaccionesvih.com;http://hivinsite.ucsf.edu).
The ideal ART regimen in renal transplant recipients has not been established, but the recommended treatment regimen should have the following characteristics:
•Sufficient potency to maintain long-term suppression of a viral load (HIV RNA) and an adequate CD4+ lymphocyte count.
•Few drug interactions with immunosuppressants that are metabolized by cytochrome p450 enzymes: calcineurin inhibitors (cyclosporine and tacrolimus), m-TOR inhibitors (sirolimus, everolimus) and corticosteroids.
•Low renal toxicity.
•An adequate cardiovascular safety profile.
•Low likelihood of producing dyslipidemia or insulin resistance (both are side effects of immunosuppressants).
The general recommendations for treating HIV infection in renal transplant recipients must be the same as those in the general population with HIV infection, but some considerations, set out i• Section 10 and summarized in Table 20, must also be taken into account7,8,313.
•NRTIs: the combination of two NRTIs (for example, TDF and FTC or ABC and 3TC) may be used safely in renal transplant recipients. With the exception of ABC, NRTIs are mainly eliminated in urine, and as such, the dose must be adjusted to renal function. TDF must be used with precaution and renal function tests must be carried out frequently. ABC must not be used in renal graft recipients from HLA-B57*01-positive donors due to the risk of drug hypersensitivity. Thymidine, AZT or d4T analogues would be contraindicated due to their high toxicity and their potential loss of viral efficacy when they are administered in combination with mycophenolate336. By contrast, there could be a synergic antiviral effect between mycophenolate and ABC, TDF or ddi337.
• NNRTIs and PIs: they can be used safely in combination with two NRTIs, but their potential drug interaction with immunosuppressants must be taken into account. In general, NNRTIs are moderate cytochrome P4503A4 inducers (which makes it necessary to increase the dose of immunosuppressants: cyclosporine, tacrolimus, sirolimus and everolimus). By contrast, PIs are potent P4503A4 inhibitors, which means that there must be a major immunosuppressant dose reduction. RPV, a new NNRTI, is a substrate and a mild inducer of CYP450 enzymes, and as such, it is expected that interactions with immunosuppressants will be clinically irrelevant338. There are currently no data on the safety and efficacy of RPV or ETR in transplant recipients. The use of any PI or NNRTI in these patients makes frequent monitoring of plasma concentrations of calcineurin inhibitors and m-TOR inhibitors essential, since they are also CYP450 and glycoprotein-P substrates. The discontinuation of PIs in a patient who receives calcineurin inhibitors or m-TOR inhibitors may cause an acute rejection of the renal graft if the doses of immunosuppressants are not increased.
• INSTI (RAL, DTG) are metabolized by UDGT-1 and, as a result, they do not interact with immunosuppressants at the cytochrome p450 level. The experience in renal transplantation of the combined use of RAL and two NRTIs has been satisfactory to date339 and it is likely that their use will decrease the high rate of renal graft rejection that existed in these patients probably related to PI or NNRTI drug interactions with immunosuppressants302,340. EVG, another INSTI soon to be available, is metabolized by CYP450 and is administered with COBI, a potent cytochrome P450 inhibitor; therefore, it will have the same pharmacokinetic interactions as PIs boosted with RTV.
• Fusion inhibitors (enfuvirtide or T20) may also be an alternative in combination with two NRTIs, but their subcutaneous administration, which is very poorly tolerated by patients, is a limitation.
• CCR5 co-receptor antagonists: MVC is a cytochrome p450 substrate and its levels may be modified by inducers or inhibitors. MVC does not alter plasma levels of immunosuppressants. Experimental studies suggest that MVC may play an important role as an anti-graft rejection drug and there are currently clinical trials underway that are attempting to evaluate this hypothesis.
In conclusion, if there is no contraindication, the ART regimen of choice in renal transplant recipients would include the combination of ABC (or TDF as an alternative) and 3TC/FTC along with RAL (or EFV as an alternative). The use of fixed dose combinations of antiretroviral drugs, “combos”, may be contraindicated in renal transplant recipients with renal function deterioration, since the drug doses cannot be adjusted. It is recommended to carry out frequent viral load tests at the earliest period after transplantation and change the ART regimens on the basis of resistance tests.
• Section 13.2.1.2 recommendations:
1. HLA B57-01 typing should be carried out in transplant donors and recipients. If positive, treatment with ABC will be avoided in the organ recipient due to the risk of hypersensitivity to the drug. (Quality of the evidence: Low. Recommendation grade: Strong).
2. It is recommended to introduce ART as soon as possible after transplantation. (Quality of the evidence: Low. (Recommendation based on consensus).
3. If there is no contraindication or risk of virologic failure, the antiretroviral regimen of choice in renal transplant recipients would include the combination of ABC (or TDF adjusted to the GFR as an alternative) plus 3TC (or FTC adjusted to the GFR) plus RAL/DTG (or EFV as an alternative). (Quality of the evidence: Moderate. Recommendation grade: Strong).
4. If NNRTIs must be used, since they are cytochrome CYP450 inducers, it will be necessary to increase the dose of immunosuppressants: cyclosporine, tacrolimus, sirolimus and everolimus. (Quality of the evidence: High. Recommendation grade: Strong).
5. If PIs/r must be used, since they are potent cytochrome P4503_A4 inhibitors, it will be necessary to reduce the dose of immunosuppressants: cyclosporine, tacrolimus, sirolimus and everolimus. (Quality of the evidence: High. Recommendation grade: Strong).
6. It is recommended to carry out more frequent viral load tests in the initial period after transplantation (3-6 months). (Quality of the evidence: Low. Recommendation based on consensus).
13.2.1.3. Pre-transplant vaccinations
See section 7.7.1 (Vaccinations in patients with chronic kidney disease and HIV infection) and Table 16, which lists the vaccines recommended in HIV-infected patients on dialysis.
Due to the increased risk of infections after transplantation, it is important to prevent them as far as possible, with the following immunizations being recommended (in addition to the regular vaccination schedule immunizations): Haemophilus influenzae b, hepatitis B (in all patients without immunity), Streptococcus pneumoniae (in patients not vaccinated or those vaccinated more than three years ago), flu (yearly, at the start of Autumn), chicken pox in seronegative patients (delaying transplantation by one month) and hepatitis A. The regimens may be consulted on the SEIMC GESIDA website http://www.gesida-seimc.org/. In addition, the diagnosis and treatment of latent infections (including tuberculosis) is important.
• Section 13.2.1.3 recommendations:
1. HIV-infected CKD patients who are candidates for receiving a renal transplant must be vaccinated against Haemophilus influenzae b, hepatitis B (in all patients without immunity), Streptococcus pneumoniae (in patients not vaccinated or those vaccinated more than three years ago), flu (yearly, at the start of Autumn), chicken pox in seronegative patients (delaying transplantation by one month) and hepatitis A. (Recommendation based on consensus).
13.2.2. Post-transplant period
13.2.2.1. Regimens for the immunosuppressants of choice in renal transplant recipients
When transplantation began in HIV-infected patients, there was fear that immunosuppressant drug use may accelerate immunological deterioration and progression to AIDS and death. Despite this fear, the experience accumulated to date has highlighted that the use of immunosuppressants in well-controlled HIV-infected patients has not led to increased susceptibility to opportunistic infections or tumours. Furthermore, it is known that some of these drugs have shown a beneficial effect on in vitro HIV replication and a potential beneficial effect on immune reconstitution341. No specific immunosuppressant treatment regimens exist for patients with HIV infection and the immunosuppressant treatment regimens used in renal transplant recipients in the ART era are not significantly different to those used in patients without HIV infection. The immunosuppressant treatment of choice in HIV-infected patients are monoclonal anti-lymphocyte antibodies (basiliximab) as induction therapy and the combination of tacrolimus with mycophenolate mofetil and corticosteroids as maintenance treatment313. On the basis of this clinical experience, some specific recommendations can be made for HIV-infected patients (Table 25).
Calcineurin inhibitors (neoral cyclosporine and tacrolimus/extended-release tacrolimus): no studies have directly compared the effect of cyclosporine with that of tacrolimus in the course of HIV infection. Cyclosporine has been the most used drug to date probably due to there being evidence of its antiviral and immunomodulating effects341, but there is currently a preference for using tacrolimus as the drug of choice due to its greater immunosuppressant potency and its lower rate of acute rejection302. These are nephrotoxic drugs whose levels require close monitoring and it is necessary to monitor interactions with antiretroviral drugs. PIs inhibit their metabolism, and as such their levels increase greatly (risk of nephrotoxicity). By contrast, NNRTIs are inducers, and as such they reduce their levels (risk of acute rejection).
Proliferation inhibitors (mycophenolate mofetil and mycophenolate sodium): they have an effect on HIV replication and are synergic with some NRTIs342. The most common side effects are diarrhoea and digestive complaints, leukocytopenia and anemia. It is necessary to monitor the level of leukocytes.
M-TOR inhibitors (sirolimus, everolimus): experimental studies343 suggest that sirolimus does not seem to have negative effects on HIV-infected patients. Sirolimus reduces CCR5 levels in CD4 cells, it inhibits the replication of HIV by R5 and increases the antiretroviral activity of fusion inhibitors and CCR5 antagonists. There is less experience compared with cyclosporine and tacrolimus. The most common side effects are leukocytopenia, anemia, acne, proteinuria, edema, skin sores, hypercholesterolemia, delayed scarring of injuries and drug pneumonitis. Its antiproliferative effect may be useful in patients with a history of Kaposi’s sarcoma before or after transplantation344. These drugs are metabolized by cytochrome P450 and they are substrates of P-glycoprotein, and their profile of interaction with antiretroviral drugs is similar to that of cyclosporine and tacrolimus.
Anti-CD25 monoclonal anti-lymphocyte antibodies (basiliximab): this antibody with anti-interleukin-2 receptor activity has been shown to increase the CD4+ lymphocyte count345 and clinical experience has not shown negative effects in HIV-infected patients.
Polyclonal anti-lymphocyte antibodies (anti-thymocyte globulin or thymoglobulin): these are potent immunosuppressants whose use is controversial due to their having been associated with severe and extended lymphocytopaenia with a higher incidence of severe infectious complications and graft loss302,346. In other studies, the results have not been so unfavourable347. It is advised to use them in patients with a high immunological risk in reduced doses and with close lymphocyte (CD3) monitoring in order to adjust the dose.
Anti-CD20 monoclonal anti-lymphocyte antibodies (rituximab): experience in renal transplant recipients with HIV infection is limited. They are indicated as pre-transplantation desensitization treatment (renal transplantation in hypersensitive or ABO-incompatible patients) and in the treatment of acute humoral rejection. However, no negative effect on HIV outcome has been observed with rituximab use in HIV-infected patients with lymphoma. There is too little experience to date for recommendations to be made348. Their use results in the additional risk of tuberculosis development.
It is advised to create teams of Nephrology and HIV-Infectious Disease specialists who will together assess the potential pharmacokinetic and clinical consequences of any modification of treatment, both in the field of antiviral efficacy and in the field of immunosuppression, due to the complexity of potential interactions between antiretroviral drugs and immunosuppressants. It is recommended to begin ART as soon as possible after transplantation.
• Section 13.2.2.1 recommendations:
1. The immunosuppressant treatment of choice in HIV-infected patients are monoclonal anti-lymphocyte antibodies (basiliximab) as induction treatment and the combination of tacrolimus and mycophenolate mofetil (or mycophenolic acid) and corticosteroids as maintenance treatment. (Quality of the evidence: Low. Recommendation grade: Recommendation based on consensus).
2. Polyclonal anti-lymphocyte antibodies must be used with caution in low doses adjusted to CD3 levels, due to an increased risk of developing prolonged lymphocytopenia and infection. Likewise, they must be assessed individually in high immunological risk renal transplantation or in treatment of severe acute rejection or corticosteroid resistance. (Quality of the evidence: Low. (Recommendation based on consensus).
13.2.2.2. Drug interactions between antiretroviral drugs and immunosuppressants
Some antiretroviral drugs may inhibit or induce metabolism of some immunosuppressants due to interference of the CYP3A4 isoenzyme of the p450 enzyme system (used in the metabolism of cyclosporine, tacrolimus, sirolimus or everolimus) (Table 25). Close monitoring of immunosuppressant treatment levels in renal transplantation patients with ART is essential. Any change in the dosage of these drugs or the introduction of new treatment must make the monitoring of immunosuppressant drug levels essential.
The use of non-immunosuppressants that are also inducers or inhibitors of the p450 enzyme system may influence the bioavailability of antiretroviral drugs, with the resulting loss of efficacy or toxicity. It is recommended to consult the potential interactions of a new treatment in HIV-infected patients on ART. For this purpose, there are different web portals with free access that analyse these interactions. They are continuously updated: (http://www.hiv-druginteractions.org; http://www.interaccionesvih.com;http://hivinsite.ucsf.edu).
• Section 13.2.2.2 recommendations:
1. PIs/r are very potent enzyme inhibitors of cyclosporine, tacrolimus, sirolimus and everolimus metabolism, which significantly increase their plasma concentrations, and as such, there is an increased risk of toxicity. A dose reduction in these immunosuppressant drugs is required. (Quality of the evidence: High. Recommendation grade: Strong).
2. NNRTIs are moderate enzyme inducers of cyclosporine, tacrolimus, sirolimus and everolimus metabolism, which significantly reduce their plasma concentrations, and as such, there is an increased risk of acute rejection. A dose increase in these immunosuppressant drugs is required. (Quality of the evidence: High. Recommendation grade: Strong).
3. If using NNRTIs or PIs/r, plasma levels of these immunosuppressant drugs must be monitored during treatment and if there is a change in dose or discontinuation of treatment. (Quality of the evidence: Moderate. Recommendation grade: Strong).
4. On the websites available, which are updated, the potential drug interactions existing between immunosuppressant treatment, antiretroviral treatment and other drugs that are prescribed to transplant patients must be consulted. (Quality of the evidence: Low. Recommendation grade: Weak).
13.2.2.3. Adherence to treatment
After renal transplantation, patients must receive a large quantity of drugs (immunosuppressants, antiretroviral drugs, prophylaxis of opportunistic infections), which may affect adherence. In this regard, it is key to have informed and educated the patient adequately before transplantation.
13.2.2.4. Acute rejection of renal transplant in HIV-infected patients
Classically, a high frequency of acute rejection was reported in renal transplantation with HIV-infected patients (30%-40%) in comparison with patients who were not infected by HIV (15%-20%). The exact mechanism is unknown, although different causes have been suggested (dysfunction of the immune system associated with HIV, inadequate immunosuppression due to drug interaction with antiretroviral drugs, racial factors, cyclosporine use and grafts from cadaveric donors). In recent series, induction with basiliximab in combination with tacrolimus, mycophenolate and steroids has been associated with acute rejection rates similar to those of the general population302,303,330,339.
In some European and American studies, a greater incidence of delayed renal function has also been observed330. Factors such as more time on dialysis before transplantation, the high prevalence of HCV infection, drug interactions and toxicity and the proinflammatory state associated with HIV may be the causes of these findings.
• Section 13.2.2.4 recommendations:
1. Renal transplantation in HIV-infected patients entails a greater risk of acute rejection. (Quality of the evidence: Moderate. Recommendation grade: Weak).
13.2.3. Coinfection with the hepatitis C virus in the post-transplantation period. (see section 8.1)
HCV coinfection is one of the major comorbidities in HIV-infected patients. HIV and HCV share the same routes of transmission, which leads to a high prevalence of coinfection by both viruses226,349. HIV-infected patients on dialysis in Spain also have a high HCV coinfection prevalence (60%). Coinfection in these patients is associated with greater comorbidity, more difficulty in its management and less access to renal transplantation10. In cases of coinfection with HCV and HIV, renal transplantation is the main risk factor for the development of the liver disease by HCV in hemodialysis patients, with liver failure being one of the main causes of death in the long-term after transplantation in these patients350. It is believed that it is due to the fact that post-transplant immunosuppressant treatment changes the natural history of the liver disease, activates viral replication and accelerates the development of chronic liver disease. In addition, HCV facilitates the onset of some types of GN in transplant recipients that may affect graft function and survival. Furthermore, IFN treatment is contraindicated in renal transplant patients (due to the risk of acute rejection and/or acute interstitial nephropathy). All these reasons suggest the need to plan HCV treatment before transplantation.
13.3. Renal and pancreas transplant
Experience in RPT in HIV-infected patients is low351-355. Isolated experiences have not shown very positive results339. Grossi et al. reported the longest experience with 4 HIV-infected RPT recipients with somewhat better results351. Combined RPT in HIV-infected patients is considered a higher-risk technique when compared to patients without HIV infection. Though the experience is limited, there is a consensus that combined RPT may be offered in adequately selected HIV patients with type 1 DM (young people and those without excessive peripheral arteriosclerosis). Indications and contraindications of RPT in HIV-infected patients are displayed in Table 23.
• Section 13.3 recommendations:
1. RPT is an alternative in HIV-infected patients with type 1 DM and CKD on dialysis or in pre-dialysis who meet the adequate criteria. There is a greater risk of infectious or surgical complications. The indication of renal and pancreas transplantation must be individualized. (Quality of the evidence: Low. Recommendation based on consensus).
Acknowledgements
The boards of GESIDA-SEIMC, SEQC and S.E.N. are grateful for the comments and opinion of those who have contributed to enrich the content of the consensus document: Drs. Anunciación González y Ángel Chocarro (Hospital Virgen de la Concha, Zamora), Dr. Antonio Buño (Hospital Universitario La Paz, Madrid), Dr. Carmen Mar (Hospital de Galdakano, Vizcaya). We are in debt with Dra. Marina Pontello Cristelli (Hospital do Rim, Sao Paulo, Brasil) who have reviewed the English version of this guidelines.
Drs. José Luis Górriz, Alberto Martínez-Castelao, Manuel Praga, Carlos Quereda, Silvia Gràcia y Rosario Montañés are members of RETIC/REDinREN (RD12/0021/0019), ISCIII-Subdirectorate General of Evaluation and the European Regional Development Fund (FEDER). Drs Félix Gutiérrez, José R. Arribas, Pere Domingo, José A. Iribarren, José López-Aldeguer, Fernando Lozano, Esteban Martínez, Eugenia Negredo, María J. Pérez-Elías, Joaquín Portilla, Antonio Rivero, and José M.ª Miró are members of the AIDS Research Network (RIS) (ISCIII-RETIC RD12/0017) of the Ministry of Health and Consumption, Instituto de Salud Carlos III. Madrid.
Conflicts of interest
The authors affirm that they have the following conflicts of interest:
José Luis Górriz has received fees from Abbvie for meetings he attended with regard to the creation of a working group on renal disorders in HIV and he received fees for Abbvie, ViiV Healthcare, Bristol-Myers-Squibb and Merck conferences.
Félix Gutiérrez states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for the Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Janssen-Cilag and ViiV Healthcare, laboratories and received fees for carrying out educational presentations or speaking at scientific meetings organised by the Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Janssen-Cilag and ViiV Healthcarelaboratories.
Joan Carles Trullas has received fees from Abbvie for participating in the working group on renal disorders in HIV.
Piedad Arazo states that she has not received any financial support or grant related to this document. In the past, she carried out consultancy work in the following laboratories: Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; she received payment for talks for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; she received payment for writing manuscripts for Abbvie and Janssen laboratories, as well as payment for educational presentations for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
José R. Arribas has carried out consultancy work in the following laboratories: Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, Tobira and ViiV Healthcare; he was given grants for clinical research by Janssen, MSD and Gilead; he received payment for talks for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Guillermina Barril received fees from Abbvie for participating in the working group on renal disorders in HIV and received fees for consultancy and/or courses or conferences for Abbvie and Gilead Sciences,
Miguel Cervero received fees from Abbvie for participating in the working group on renal disorders in HIV, he carried out consultancy work in Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck and ViiV Healthcare, laboratories and received financial support for attending Gilead, Janssen-Cilag, Boehringer Ingelheim and Bristol-Myers Squibbconferences.
Frederic Cofán received fees from Abbvie for participating in the working group on renal disorders in HIV and received fees for consultancy and conferences from Fresenius, Novartis, Roche, Bristol-Myers-Squibb and Abbvie.
Pere Domingo received fees from Abbvie for the creation of a working group on renal disorders in HIV and he has carried out consultancy work in the following laboratories: Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck and ViiV Healthcare; he was given grants for clinical research by Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare and he received payment for talks for Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare.
Vicente Estrada states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for the Abbvie, Gilead Sciences, Janssen and MSD laboratories. He received grants for clinical research by Janssen, MSD and Abbvie and payment for talks for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Xavier Fulladosa received fees from Abbvie for participating in the working group on renal disorders in HIV, carried out consultancy work in Gilead Sciences laboratory and received payment for Gilead Sciences conferences.
María J. Galindo states that she has not received any financial support or grant related to this document. In the past, she carried out consultancy work for the Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, and Merck laboratories; she was given grants for clinical research by Abbvie, Boehringer Ingelheim, Glaxo and Janssen; she received payment for talks for Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and Roche, and collaborated in the preparation of educational material for Janssen, Pfizer, ViiV, Glaxo and Abbvie.
Silvia Gràcia received fees from Abbvie for participating in the working group on renal disorders in HIV.
José Antonio Iribarren states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for the Gilead Sciences and Janssen-Cilag laboratories; he received clinical research grants from the following laboratories and organizations: Abbvie, Bristol-Myers Squibb, the Basque Government, FIPSE (Foundation for Research and Prevention of AIDS) and FISS (Health Research Fund), and financial support for attending Abbvie, Gilead, Janssen-Cilag and ViiV conferences, and he participated in educational activities, talks or symposia sponsored by Abbvie, Bristol-Myers Squibb, Gilead, Merck, Novartis Janssen, Pfizer and ViiV.
Hernando Knobel states that he has not received any financial support or grant related to this document. In the past, he received payment for consultancy work and talks for the following laboratories: Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
José López-Aldeguer states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for the following laboratories: Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare; he was given grants for clinical research by Bristol-Myers Squibb, Merck and ViiV Healthcare. and he received payment for talks for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Fernando Lozano states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithkline, Janssen, Merck-Sharp & Dome, Pfizer, Roche Pharmaceuticals and ViiV Healthcare, and he received payment for talks for Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithkline, Jansen, Merck-Sharp & Dome, Pfizer, Roche Pharmaceuticals and ViiV Healthcare.
Alberto Martínez-Castelao received fees from Abbvie for the creation of a working group on renal disorders in HIV; he received fees for conferences for Abbvie, Amgen, Boehringer-Ingelhein). Esteve, Novartis and Roche, and he participated in consultancy work in laboratories of Abbvie, Amgen, Boehringer-Ingelheim, Esteve, Janssen-Cilag and Novartis.
Esteban Martínez states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec and ViiV Healthcare laboratories; he received payment for talks for Abbvie, Boehringer-Ingelhein). Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec and ViiV Healthcare, as well as payment for educational presentations for Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline and ViiV Healthcare.
María A. Mazuecos coordinated a research group in renal transplantation in HIV patients and received financial support for carrying out this research from Astellas Pharma.
Celia Miralles states that she has not received any financial support or grant related to this document. In the past, she carried out consultancy work for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare laboratories; she received payment for talks for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; she received payment for writing manuscripts for Abbvie, Bristol-Myers Squibb, Gilead Sciences and ViiV Healthcare laboratories, as well as payment for educational presentations for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Rosario Montañés received fees from Abbvie for participating in the working group on renal disorders in HIV.
Eugenia Negredo states that she has not received any financial support or grant related to this document. In the past, she carried out consultancy work for Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, Janssen, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme and ViiV Healthcare laboratories; she received payment for talks for Abbvie, Boehringer-Ingelheim, Roche, Janssen, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme and ViiV Healthcare laboratories, as well as payment for educational presentations for Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline and ViiV Healthcare.
Rosario Palacios states that she has not received any financial support or grant related to this document. In the past, she has carried out consultancy work in Boehringer Ingelheim laboratories and she received payment for talks for Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, ViiV Healthcare and Roche.
María J. Pérez Elías states that she has not received any financial support or grant related to this document. In the past, she carried out consultancy work for Abbott Laboratories, Bristol-MyersSquibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare laboratories; she was given grants for clinical research by Abbott Laboratories, Gilead Sciences, ViiV Healthcare and Janssen laboratories; she received payment for talks for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare, as well as payment for educational presentations for Abbott Laboratories, Bristol-Myers Squibb, Janssen, Merck Sharp Dome and ViiV Healthcare.
Joaquín Portilla received fees from Abbvie for participating in the working group on renal disorders in HIV and he has carried out consultancy work in the following laboratories: Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; he was given grants for clinical research by Abbvies, Janssen, Merck and ViiV Healthcare and he received payment for talks for Abbvies, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, Roche and ViiV Healthcare.
Manuel Praga received fees from Abbvie for participating in the working group on renal disorders in HIV and received financial support for research and fees for consultancy and conferences for Novartis, Astellas, Gambro, Fresenius, Alexion, Abbvie, Glaxo, Gilead, Roche, Merck, Janssen and Pfizer.
Carlos Quereda states that he has no conflicts of interest in this project.
Antonio Rivero has carried out consultancy work in Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare laboratories; he was given grants for clinical research by Abbvie, Gilead Sciences, Merck and ViiV Healthcare laboratories; he received payment for talks for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare, as well as payment for educational presentations for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Juan M. Santamaría received fees from Abbvie for participating in the working group on renal disorders in HIV.
Jesús Sanz states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, ViiV Healthcare and Boehringer Ingelheim laboratories; he received payment for talks for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, ViiV Healthcare and Boehringer Ingelheim and received payment for educational presentations for ViiV Healthcare.
José Sanz states that he has not received any financial support or grant related to this document. In the past, he gave informative talks and carried out consultancy work, for which he received fees from ViiV Healthcare, Bristol-Myers Squibb, Gilead Sciences, Janssen, MSD, Boehringer-Ingelheim and Abbvie.
José M. Miró received fees from Abbvie for creating a working group on renal disorders in HIV and he has carried out consultancy work in Abbvie Laboratories, Bristol-Myers Squibb, Gilead Sciences, Merck, Novartis and Sanofi laboratories; he was given grants for clinical research by Cubist, Novartis, Merck, the Health Research Fund (FIS) of the Instituto de Salud Carlos III (Madrid), the Foundation for Research and Prevention of AIDS in Spain (FIPSE, Madrid), the Ministry of Health, Social Services and Equality (MSSSI, Madrid), National Institutes of Health (NIH, Bethesda, MA, USA) and NEAT and he received payment for talks for Novartis and ViiV Healthcare.
Finally, the logistics of the meetings attended by the members of this panel who participated in a working group on renal disorders in HIV was financed by Abbvie Spain laboratories.
Table 1. Types of renal pathology in HIV-infected patients
Table 2. Prognosis of chronic kidney disease by glomerular filtration rate and albuminuria
Table 3. Potentially nephrotoxic non-antiretroviral drugs used in HIV-infected patients
Table 4. The most common clinical presentations and laboratory markers in glomerular diseases of HIV-infected patients
Table 5. Causes of acquired Fanconi¿s syndrome
Table 7. Factors associated with chronic kidney disease progression
Table 8. Basic and comprehensive renal studies that must be carried out in HIV-infected patients
Table 9. Glomerular filtration rate estimation equations (IDMS-traceable methods) and formulae for calculating fractional excretion of phosphate and urate
Table 10. Regularity of renal assessment in HIV-infected patients
Table 11. Interactions and precautions in the use of the main antihypertensive drugs in HIV-infected patients
Table 12. Causes of secondary dyslipidemia
Table 13. Dose adjustment for lipid-lowering drugs and interactions with antiretroviral drugs
Table 14. Indication for glucose lowering drugs in diabetes mellitus patients and adjustment according to eGFR
Table 15. Causes of hypophosphatemia
Table 16. Vaccines recommended in HIV-infected patients on dialysis
Table 17. Antiretroviral drugs. Dose adjustment (I)
Table 18. Antiretroviral drugs. Dose adjustment (II)
Table 20. General recommendations for antiretroviral treatment in HIV-infected patients with chronic kidney disease, those on renal replacement therapy, or those with a renal transplant
Table 21. Criteria for including renal HIV-infected patients on the renal transplantation waiting list
Table 22. Criteria for including HIV-infected patients on the renal and liver transplantation waiting list
Table 23. Criteria for including HIV-infected patients on the renal and pancreas transplantation waiting list
Table 24. Survival of HIV-infected renal transplant patients and grafts before and after the introduction of highly active antiretroviral therapy
Table 25. Immunosuppressant treatment in renal transplant recipients with HIV infection
Figure 1. Algorithm for the initial renal evaluation of HIV-infected patients.
Figure 2. Algorithm for the evaluation of renal function impairment in HIV-infected patients.
Figure 3. Algorithm for the hematuria study in HIV-infected patients.
Figure 4. Algorithm for evaluating proteinuria in HIV-infected patients.
Figure 5. Algorithm for the management of hyperglycemia in patients with chronic kidney disease and HIV infection.
Figure 6. Algorithm for the evaluation of hypophosphatemia in HIV-infected patients.
Table 19. Dose adjustment for antimicrobial drugs
ACRONYMS