Urinary levels of TWEAK (uTWEAK) may be correlated with the degree of lupus nephritis (LN) activity. Our objective was to determine the sensitivity and specificity of uTWEAK in Mexican patients with untreated active lupus nephritis.
MethodsAn exploratory study was performed; four groups of patients were analyzed as follows: 1) patients with systemic lupus erythematosus (SLE) without renal activity (SLE-LN), 2) patients with SLE with renal activity (SLE+LN), 3) patients with other types of glomerulopathy (glomerulonephritis, GMN), 4) and healthy patients (controls).
ResultsIn all, 44 patients, with an average age of 35.9±11.5 years, were evaluated. uTWEAK levels were higher in patients with SLE+LN compared with patients in the other groups: SLE+LN 12.88±8.33, SLE-LN 3.12±2.31, GMN 4.36±2.31 and controls 2.41±1.94pg/mg Cr (p=0.007). A total of 72.7% of the cases had renal activity index scores above 12, and 90.9% of the cases had scores of chronicity below 6 points. Receiver Operating Characteristic (ROC) curve analysis revealed that uTWEAK levels above 4.91pg/mg Cr had a sensitivity of 81% and a specificity of 75% for the diagnosis of renal activity due to lupus, with an area under the curve of 0.876 (95% CI: 0.75–0.99). However, no significant correlation was observed between the levels of uTWEAK and the histological findings specific to the activity and chronicity associated with SLE.
ConclusionsOur study revealed that uTWEAK can adequately distinguish renal activity due to lupus, but cannot predict the degree of histological activity in Mexican patients with active lupus nephropathy.
Los niveles urinarios de TWEAK (uTWEAK) pueden correlacionarse con el grado de actividad de nefritis lúpica (NL). Nuestro objetivo fue determinar la sensibilidad y especificidad de los uTWEAK en pacientes mexicanos con NL activa sin tratamiento farmacológico previo.
MetodologíaSe realizó un estudio exploratorio en el que se incluyeron 4 grupos de pacientes: 1) pacientes con lupus eritematoso sistémico sin actividad renal (LES-NL); 2) pacientes con lupus eritematoso sistémico con actividad renal (LES+NL); 3) pacientes con otras glomerulopatías y 4) controles sanos.
ResultadosLa edad promedio de los 44 pacientes fue de 35,9±11,5 años. Los uTWEAK fueron más elevados en pacientes con LES+NL comparados con los otros grupos: LES+NL (12,88±8,33), LES-NL (3,12±2,31), otras glomerulopatías (4,36±2,31) y grupo control (2,41±1,94pg/mgCr) (p=0,007). En el 72,7% de los casos se observó un índice de actividad renal mayor a 12 puntos y en el 90,9% de los casos los índices de cronicidad estaban por debajo de 6 puntos. La curva ROC reveló que los niveles urinarios por encima de 4,91pg/mg Cr tienen sensibilidad del 81% y especificidad del 75% para el diagnóstico de actividad renal secundaria a lupus, con área debajo de la curva de 0,876 (IC 95%: 0,75-0,99). Sin embargo, no se observó correlación significativa entre los uTWEAK y los hallazgos histológicos específicos de actividad y cronicidad asociados a LES.
ConclusionesNuestro estudio revela que los uTWEAK pueden distinguir adecuadamente actividad renal secundaria a lupus, pero no predicen el grado de actividad histológica en pacientes mexicanos con NL activa.
Lupus nephritis (LN) is a severe complication in patients with systemic lupus erythematosus (SLE).1 It has been demonstrated that LN diminishes the short-term and long-term survival and quality of life of patients.2,3 A percutaneous kidney biopsy is considered the gold standard in the evaluation of disease activity in the kidneys due to lupus. However, as this method is invasive, not all patients are able to undergo this procedure.3,4
The levels of proteinuria and serum creatinine have been used to non-invasively evaluate kidney activity due to lupus,5 but the relevance of these values has been questioned due to their low predictive values.6 In the same vein, antibodies (e.g., anti-DNA, anti-nuclear antibodies, and anti-Smith antibodies) and levels of serum complement (C3 and C4) have been used to evaluate kidney activity due to lupus.7,8 However, due to low sensitivity and specificity, these have not been considered adequate biomarkers for SLE renal activity.9–11 For this reason, a search has been undertaken for new biomarkers that might allow for the diagnosis and monitoring of patients with active LN.12 Studies of mouse models have demonstrated that tumor necrosis factor-like weak inducer of apoptosis (TWEAK) may be associated with renal activity due to lupus.13,14 TWEAK is a member of the superfamily of tumor necrosis factors, which are involved in multiple physiological processes related to cellular proliferation, migration, survival, differentiation, and apoptosis13; TWEAK regulates these processes through Fn14 binding to TWEAK leads to TRAF2 and TRAF5 recruitment and activation of intracellular signaling pathways. The specific signalling pathways activated by TWEAK/Fn14 system and the outcome of TWEAK binding depend on cell type, cell state and the microenvironment.14
TWEAK has a proliferative effect in quiescent renal tubular cells; TWEAK-induced tubular cell proliferation requires ERK1/2, p38 MAPK, phosphoinositide 3-kinase/Akt and NFκB in a manner that disruption of one of them prevents proliferation.15
TWEAK alone does not promote renal tubular cell apoptosis. However, co-stimulation with sensitizing agents present in injured kidneys, such as TNFα and interferon-γ, leads to tubular cell apoptosis.16
Podocytes are also TWEAK responsive. TWEAK stimulation of human and murine podocytes induces a proinflammatory response via the activation of the NFκB signaling pathway.17
In animal models, TWEAK/fn14 and the development of lupus nephritis have been showed. In Fn14 knockout MRL/lpr mice, were markedly lower levels of proteinuria compared with wild-type MRL/lpr mice, and Fn14 knockout mice showed significantly improved renal histopathology accompanied by attenuated glomerular and tubulointerstitial inflammation these findings indicate that TWEAK/Fn14 plays an important role in the pathogenesis of lupus.18
Subsequent clinical studies have indicated the utility of urinary levels of TWEAK in the diagnosis of active LN.19,20,21 However, in these studies, the quantification of TWEAK was performed in patients who had already received immunosuppressive treatment, which implies a high possibility that the urinary expression of TWEAK in these patients was modified.21–23 On the contrary, earlier published studies had not included Mexican patients, which comprise a population that is known to experience SLE with a higher degree of clinical severity and with limited therapeutic response.24–26 As a result, the objective of our study was to determine the utility of urinary levels of TWEAK as a biomarker of lupus renal activity in recently diagnosed, previously untreated Mexican patients.
Materials and methodsStudy designThis was a clinical study in which all patients were Mexican and were admitted to the Dr. Eduardo Liceaga Hospital General de Mexico. The recruitment period spanned from July 1, 2013 to June 20, 2015. This study was conducted in accordance with the Declaration of Helsinki and was approved by the hospital's Ethics and Health Research Committee (registration code: DI/15/105/4/018).
PopulationPatients with SLE were selected from both outpatient and inpatient clinics according to the following inclusion criteria: 1) patients who were recently diagnosed with SLE according to the criteria defined by The Systemic Lupus International Collaborating Clinics24 and who did not receive previous immunosuppressive treatment; 2) patients with clinical or histological criteria of primary glomerulopathies who were recently diagnosed and who did not undergo prior pharmacological treatment; and 3) clinically healthy volunteer participants.
The selected population was divided into four groups as follows: 1) patients with active SLE with renal activity corroborated through kidney biopsy (SLE+LN); 2) patients with SLE without evidence of renal activity (SLE-LN); 3) patients with primary glomerulopathies (GMN); and 4) clinically healthy volunteers (controls) (see Fig. 1).
Patients were excluded if they were diagnosed with active infections, neoplasms, or any other type of autoimmune disease unrelated to lupus. In addition, patients were excluded if their kidney tissue samples were insufficient for diagnosis. A confidence level of 95% as well as a statistical power of 80%, this resulted in a sample size of at least 11 participants per group.
Execution of the studyPatients who fulfilled the selection criteria for this study were invited to participate, and those who accepted then signed an informed consent and a permission form for their data to be published. Patients were tested for antinuclear antibodies (ANA), anti-Smith antibodies (anti Sm), anti-DNA antibodies, and levels of complement (C3 and C4). Routine blood chemistry and a urine test strip were also performed. In addition, glucose, urea, creatinine, sodium, potassium, chlorine, microscopic urinary sediment, and the levels of 24-h urinary protein were evaluated. In addition to these tests, a chronic viral hepatitis panel was run (Hepatitis B and C viruses), and anti-HIV antibodies, anti-cardiolipin antibodies (IgG and IgM), anti-beta 2 glycoprotein, lupus anticoagulant, anti-Ro antibodies, and anti La, c-ANCA and p-ANCA were assessed. In the healthy volunteers, a urine test strip was performed, the patients with protein major to zero recollected 24-h urine protein; other serum parameters like other groups were determinate. In the case of patients who underwent renal biopsies, coagulation times (PT, PTT, and INR) were tested prior to the procedure.
Measurement of uTWEAKTWEAK levels in urine were determinate by human TWEAK Instant ELISA Kit from eBioscience (BMS2006INST) briefly, Added 50μl of each sample, in duplicate, to the designated wells and mixed the contents, Covered with an adhesive film and incubated at room temperature (18°C–25°C) for 3h, Removed adhesive and Washed the microwell strips 6 times with approximately 400μl Wash Buffer, Pipetted 100μl of TMB Substrate Solution to all wells, Incubated the microwell strips at room temperature (18°C–25°C) for about 10min.
Stopped the enzyme reaction with 100μl of Stop Solution, results read immediately after the Stop Solution was added on a spectrophotometer using 450nm. TWEAK levels were derived from an average of the duplicate assays that were performed for all samples. All assays were performed in a blinded fashion, without knowledge of the patient's identity, disease diagnosis, or disease severity. To account for variations in urine concentration, the uTWEAK levels were normalized to urine creatinine levels. Corrected uTWEAK levels are therefore expressed as picograms per milligram of creatinine (pg/mg Cr).
StatisticsIn regards to data analysis, the software program Statistical Package for the Social Sciences (SPSS) version 19 was used. The means and standard deviations or the medians and interquartile ranges were used to summarize the characteristics of the samples. Categorical variables were summarized with frequencies and percentages. Pearson or Spearman correlation coefficients were established with the continual values of urinary TWEAK, histological findings, and the SLEDAI (Systemic Lupus Erythematous Disease Activity Index) score, depending on the distribution of the variables. ROC curves were calculated to establish sensitivity, specificity for diagnosis of renal activity due lupus nephritis. A value of p<0.05 was considered significant.
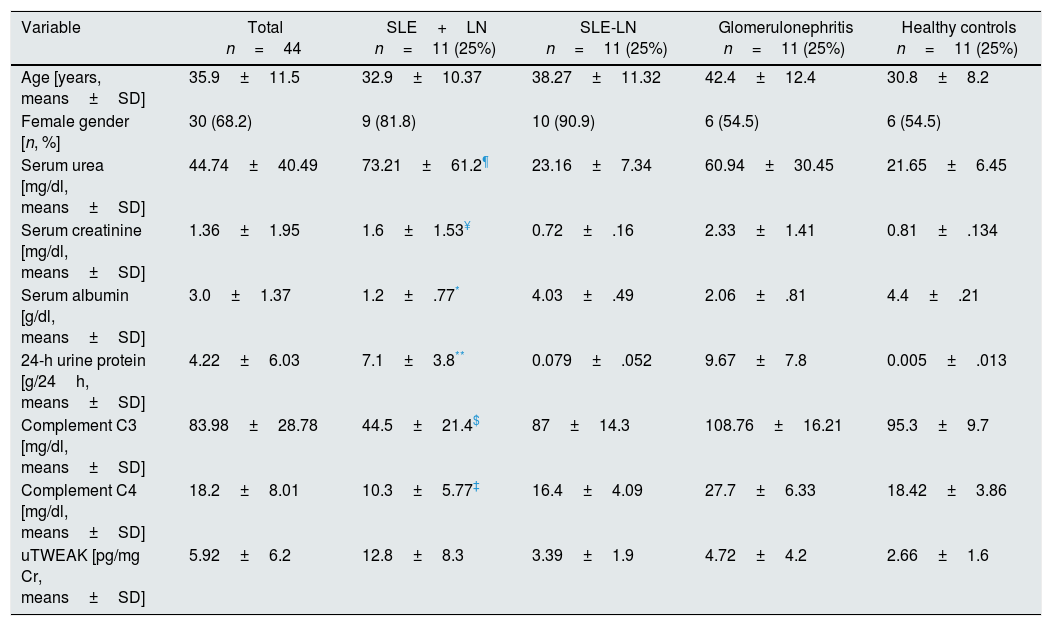
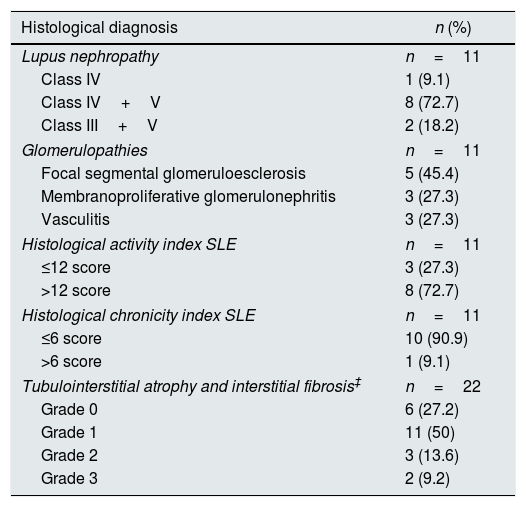
ResultsBaseline and histological characteristics of the study populationThe baseline characteristics of the population may be observed in Table 1. In all, 44 patients who were distributed within four groups were included in the study (Fig. 1). Participants had an average age of 35.9±11.5 years, and as 68.2% of the participants were women, they were predominant in all the groups. Table 2 presents the histological characteristics of the patients who underwent kidney biopsy. In the group of patients with LN, histological classes IV+V dominated (72.7%) and 72.7% of the patients presented indices of histological activity above 12 points; moreover, 90.9% of the patients who underwent a biopsy presented less than 6 points on the chronicity index. In the group of patients with glomerulopathies, five cases were of focal and segmental glomeruloesclerosis (45.5%), three were of vasculitis (27.3%) and three were of primary membranoproliferative glomerulonephritis (27.3%).
Baseline characteristics of the study population.
| Variable | Total n=44 | SLE+LN n=11 (25%) | SLE-LN n=11 (25%) | Glomerulonephritis n=11 (25%) | Healthy controls n=11 (25%) |
|---|---|---|---|---|---|
| Age [years, means±SD] | 35.9±11.5 | 32.9±10.37 | 38.27±11.32 | 42.4±12.4 | 30.8±8.2 |
| Female gender [n, %] | 30 (68.2) | 9 (81.8) | 10 (90.9) | 6 (54.5) | 6 (54.5) |
| Serum urea [mg/dl, means±SD] | 44.74±40.49 | 73.21±61.2¶ | 23.16±7.34 | 60.94±30.45 | 21.65±6.45 |
| Serum creatinine [mg/dl, means±SD] | 1.36±1.95 | 1.6±1.53¥ | 0.72±.16 | 2.33±1.41 | 0.81±.134 |
| Serum albumin [g/dl, means±SD] | 3.0±1.37 | 1.2±.77* | 4.03±.49 | 2.06±.81 | 4.4±.21 |
| 24-h urine protein [g/24h, means±SD] | 4.22±6.03 | 7.1±3.8** | 0.079±.052 | 9.67±7.8 | 0.005±.013 |
| Complement C3 [mg/dl, means±SD] | 83.98±28.78 | 44.5±21.4$ | 87±14.3 | 108.76±16.21 | 95.3±9.7 |
| Complement C4 [mg/dl, means±SD] | 18.2±8.01 | 10.3±5.77‡ | 16.4±4.09 | 27.7±6.33 | 18.42±3.86 |
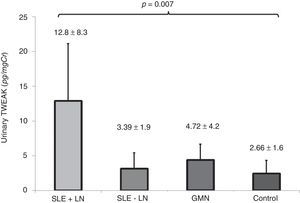
| uTWEAK [pg/mg Cr, means±SD] | 5.92±6.2 | 12.8±8.3 | 3.39±1.9 | 4.72±4.2 | 2.66±1.6 |
Renal histopathological findings.
| Histological diagnosis | n (%) |
|---|---|
| Lupus nephropathy | n=11 |
| Class IV | 1 (9.1) |
| Class IV+V | 8 (72.7) |
| Class III+V | 2 (18.2) |
| Glomerulopathies | n=11 |
| Focal segmental glomeruloesclerosis | 5 (45.4) |
| Membranoproliferative glomerulonephritis | 3 (27.3) |
| Vasculitis | 3 (27.3) |
| Histological activity index SLE | n=11 |
| ≤12 score | 3 (27.3) |
| >12 score | 8 (72.7) |
| Histological chronicity index SLE | n=11 |
| ≤6 score | 10 (90.9) |
| >6 score | 1 (9.1) |
| Tubulointerstitial atrophy and interstitial fibrosis‡ | n=22 |
| Grade 0 | 6 (27.2) |
| Grade 1 | 11 (50) |
| Grade 2 | 3 (13.6) |
| Grade 3 | 2 (9.2) |
According to the sex in all groups, we found differences in serum creatinine, c4 levels and uTWEAK levels (supplementary Table 1). In patients with SLE we found statistical differences in uTWEAK levels accord the sex, with major levels in female group 9.05±7.8 vs. 2.34±1.76, p=0.005.
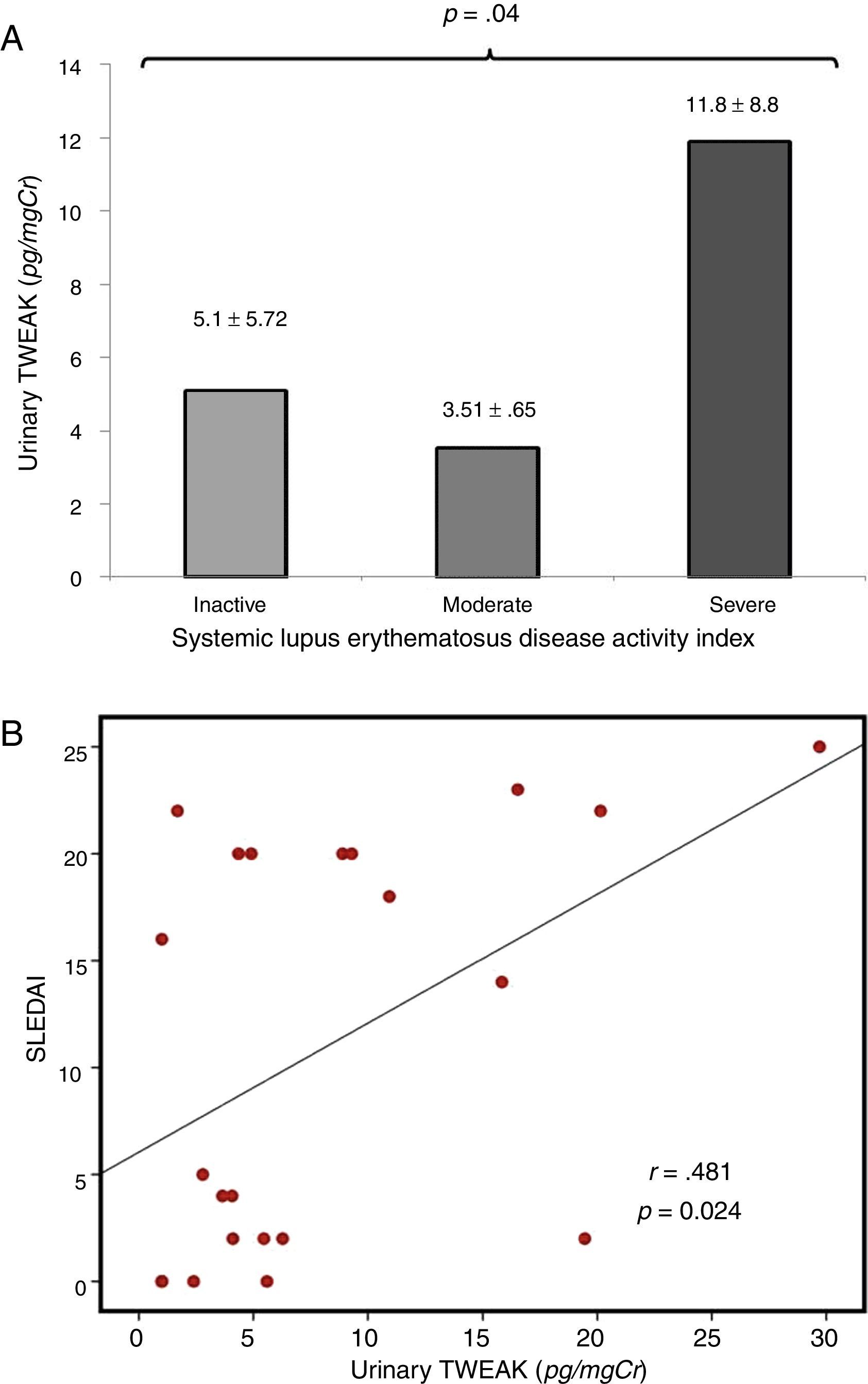
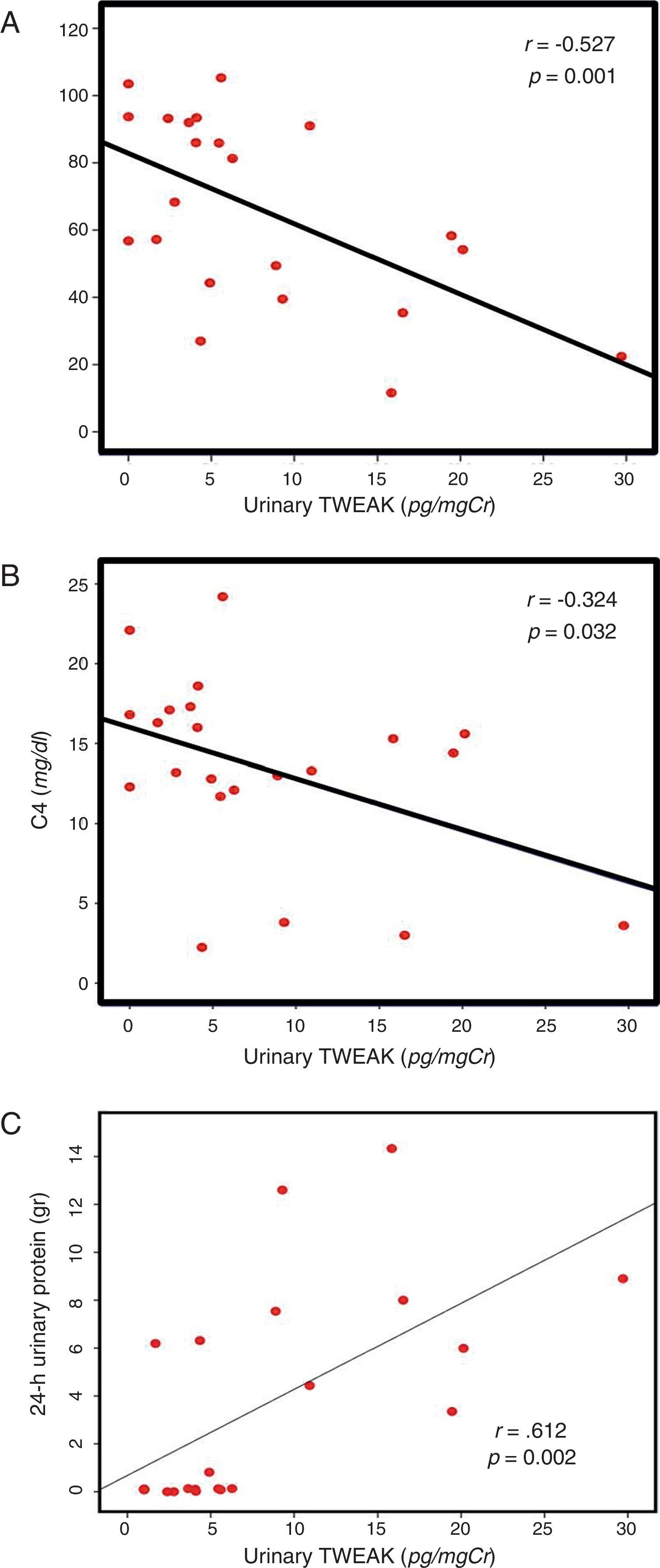
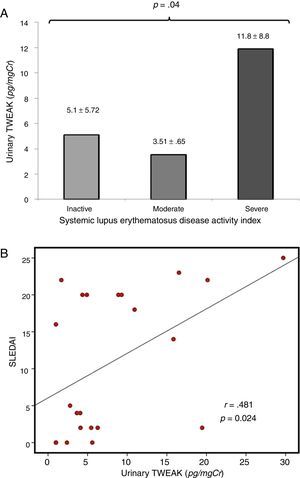
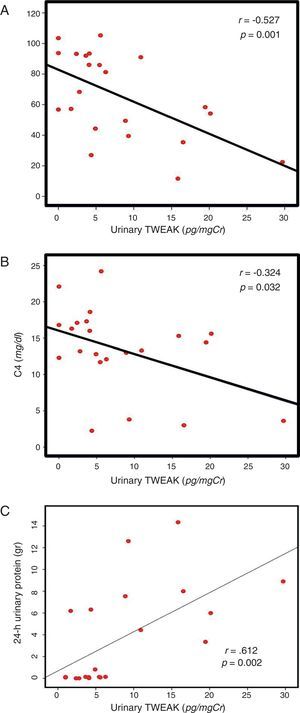
Correlation of uTWEAK with clinical markers of SLEIn patients with lupus, the levels of urinary TWEAK were measured according to the SLEDAI score and were graphed in three categories as follows: 1) patients with at least 3 activity points (without activity); 2) patients between 3 and 12 points of activity (moderate activity); and 3) patients with more than 12 activity points (severe activity). The group of patients with severe disease activity as measured by the SLEDAI presented higher levels of uTWEAK with statistically significant differences between groups (p=0.04) (Fig. 2A); moreover, a directly proportional correlation was observed between the levels of uTWEAK and the SLEDAI score (r=0.481, p=0.024) (Fig. 2B). Additionally, a direct inverse correlation was documented between the levels of uTWEAK and the C3 (r=−0.527, p<0.001) and C4 complement fractions (r=−0.324, p=0.032) (Fig. 3A and B respectively). In contrast, the levels of proteinuria showed a directly proportional correlation to the values of uTWEAK (r=0.612, p=0.002) (Fig. 3C); however, a correlation was not observed between the levels of uTWEAK and the levels of serum creatinine (r=−0.151, p=0.328) and serum urea (r=−0.026, p=0.865).
(A) uTWEAK levels according to the SLEDAI index. The bar with horizontal lines (3 patients) shows patients with inactive systemic lupus erythematosus (SLEDAI less than 3) (uTWEAK 5.1±5.72); the bar with fine dots (9 patients) indicates patients with moderate systemic lupus erythematosus activity (SLEDAI between 4 and 15) (uTWEAK 3.51±0.653); the gray (10 patients) bar indicates patients with severe systemic lupus erythematosus activity (SLEDAI greater than 15) (uTWEAK 11.89±8.85). A comparison of the groups showed significant differences (p=0.04) with higher uTWEAK levels in patients with severe activity. (B) Correlation between uTWEAK levels and SLEDAI score. A correlation analysis revealed a directly proportional correlation between the levels of uTWEAK and the SLEDAI score (r=0.481, p=0.024).
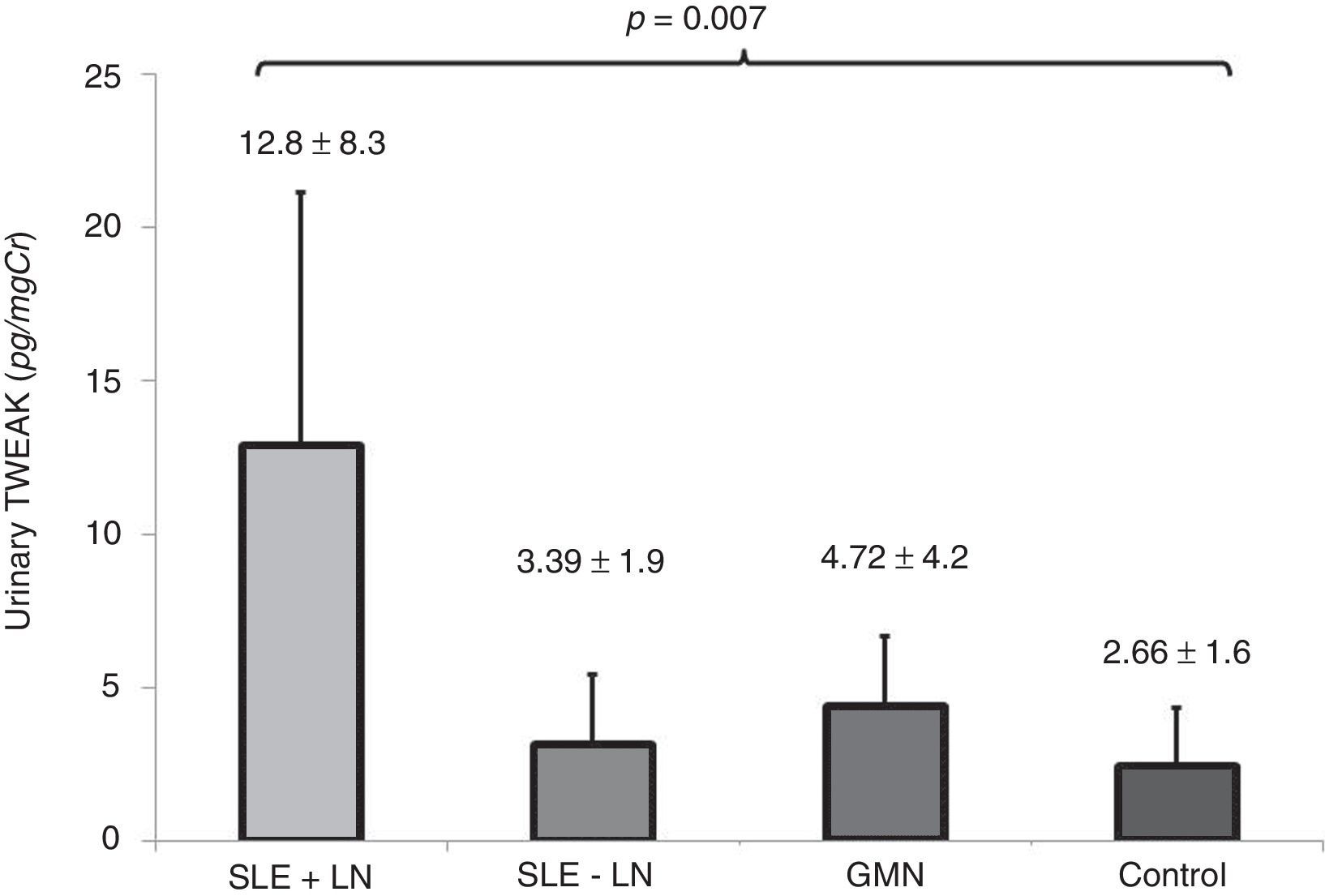
The levels of uTWEAK in patients in each of the groups are shown in Fig. 4. Patients with active lupus nephropathy were determined to have 12.8±8.33pg/mg Cr uTWEAK, whereas patients with SLE without renal activity were found to have 3.12±2.31pg/mgCr uTWEAK. Patients with other glomerulopathies were determined to have 4.36±2.31pg/mg Cr uTWEAK, while controls were found to have 2.41±1.94pg/mg Cr uTWEAK. A significant difference was observed among the groups (p=0.007). Significantly higher levels of uTWEAK were observed when the group of patients with other glomerulopathies was compared with healthy controls (GMN: 4.36±2.31 vs. control: 2.41±1.94, p=0.012) and with patients with SLE without renal activity (GMN: 4.36±2.31 vs. SLE-LN: 3.12±2.3, p=0.030). When we compared the levels of uTWEAK in patients with glomerulonephritis with the levels of uTWEAK in patients with lupus-related renal activity, a statistically significant difference was observed (GMN: 4.36±2.31 vs. LES+NL: 12.88±8.33, p<0.001).
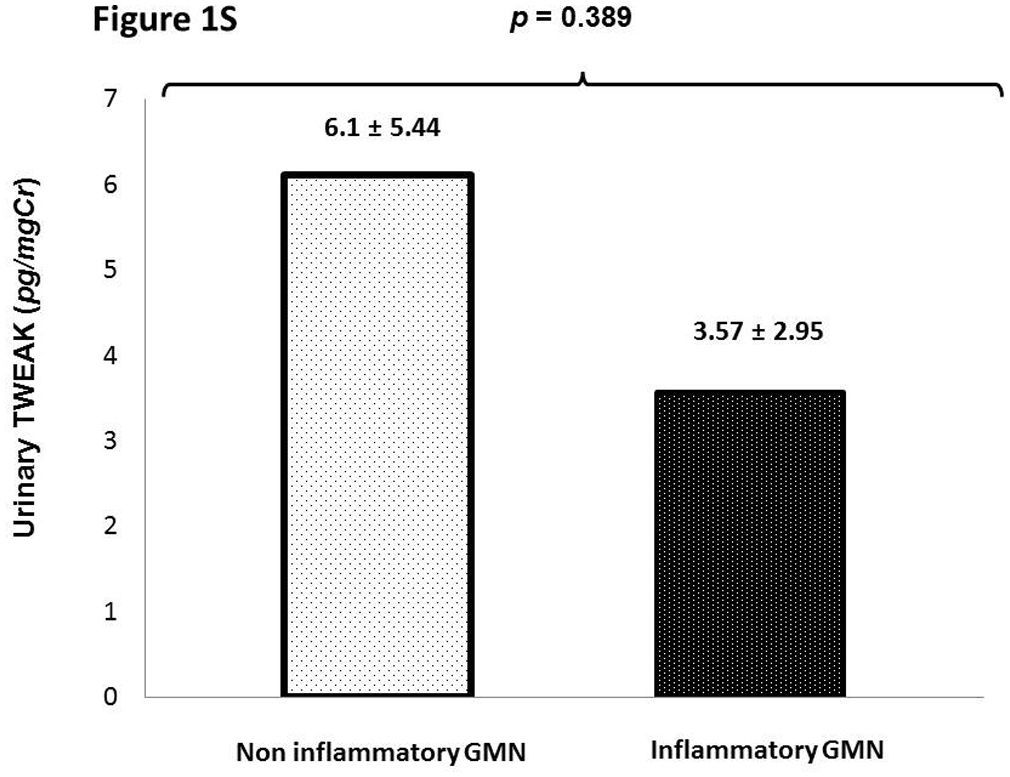
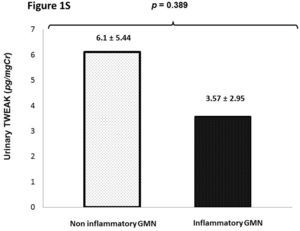
In patients with glomerulonephritis we did not find significance statistical in the uTWEAK levels when evaluated accord the etiology of glomerulonephritis (inflammatory GMN vs. non inflammatory GMN) 6.1±5.44 vs. 3.57±2.95, p=0.389 (Supplementary figure 1S).
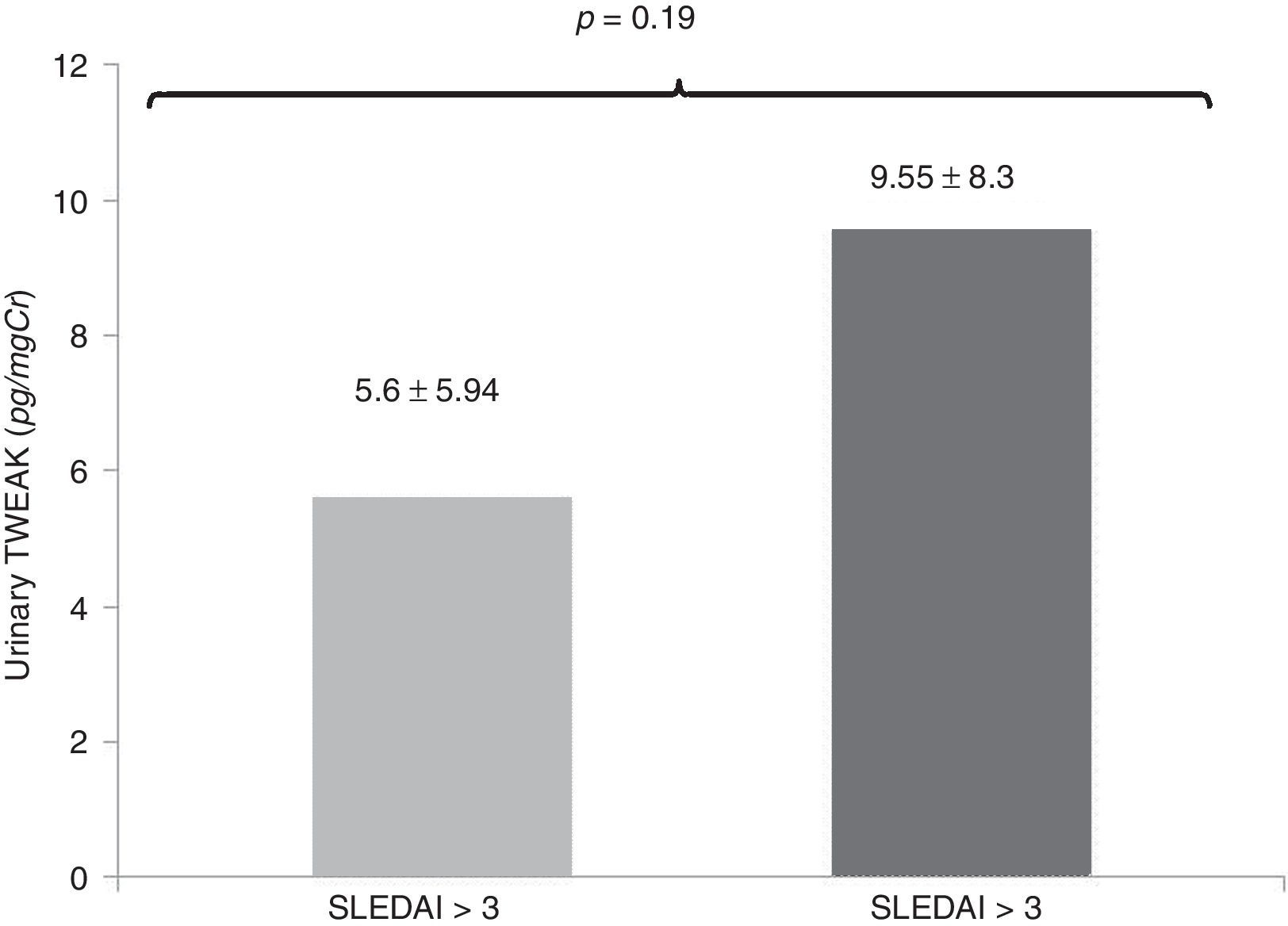
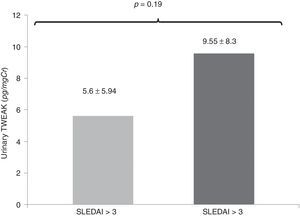
In an additional analysis, in the comparison of patients with active SLE (SLEDAI score above 3), the levels of uTWEAK were greater in the group of patients with active lupus compared with patients with SLE without activity without statistical significance (active SLE: 9.55±8.3, vs. SLE without activity: 5.6±5.94pg/mg Cr, p=0. 19) (Fig. 5).
Comparison of the levels of uTWEAK in patients with active SLE (SLEDAI score above 3). The levels of uTWEAK were higher in patients with active lupus compared with patients with SLE without activity; active SLE (gray bar 9.55±8.3 (14 patients) vs. inactive LES (black bar): 5.6±5.94pg/mgCr (8 patients) (p=0.19).
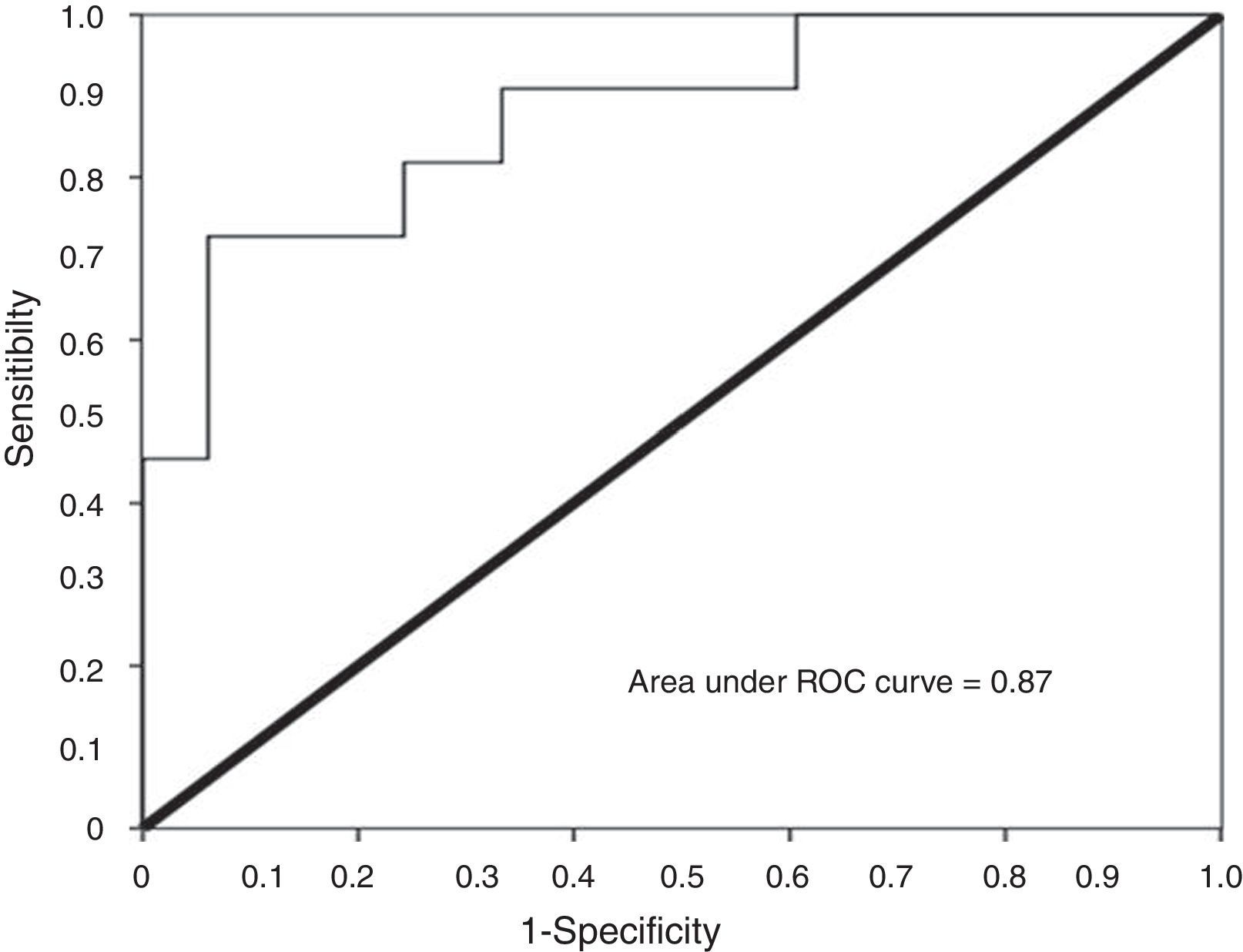
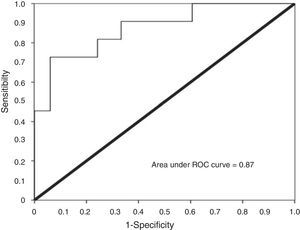
Using urinary TWEAK levels above 4.91pg/mg Cr as a cut-off point, the ROC curve analysis showed a sensitivity of 81% and a specificity of 75%, with an area under the curve of 0.87 (CI95% 0.75–0.99) (Fig. 6).
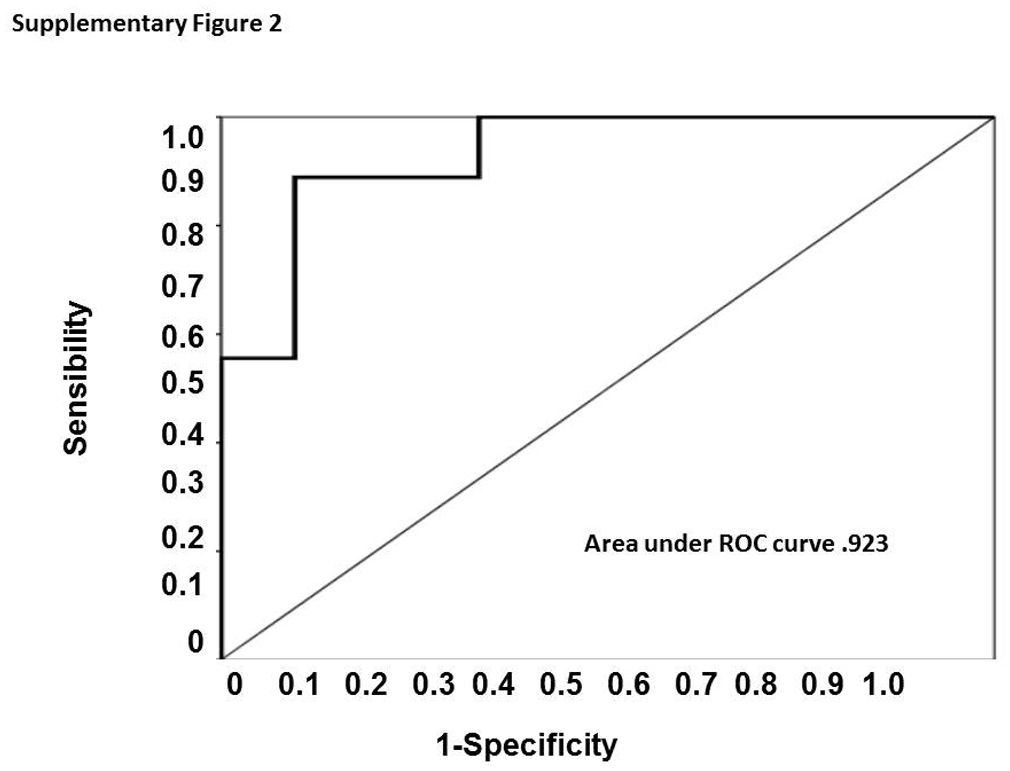
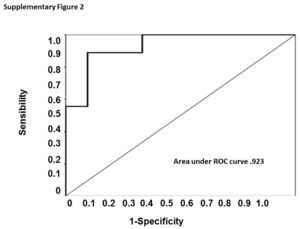
When evaluated the urinary TWEAK levels in female group, we establish a cut-off point of 7.59pg/mg Cr and the ROC curve showed a sensitivity of 88% and specificity of 90% with an area under the curve of 0.93 (CI 0.940–1.0), however in the male group we could not determinate a cut-off point with an adequate sensibility and specificity. (Supplementary figure 2).
DiscussionThe effectiveness of the levels of proteinuria and serum creatinine in the evaluation of renal activity due to lupus has been questioned.10 Additionally, the sensitivity and specificity of immunological markers (e.g., levels of complement, anti-DNA antibodies, ANA and anti-Sm antibodies) that are used to diagnose active lupus nephritis are low.8,21 Due to the absence of specific biomarkers for LN, an interest arose to find new biomarkers that might allow for the sensitive and specific diagnosis of renal lupus activity.8,12 Our results demonstrated the efficacy of the quantification of uTWEAK levels in lupus nephritis in Mexican population, where the patients, who were recently diagnosed with active lupus, did not receive prior immunosuppressive treatment.
Studies have evaluated the levels of uTWEAK as a biomarker of LN.21–23 Schwartz et al. observed significantly higher levels of uTWEAK in patients with LN in comparison with the levels of uTWEAK in patients with SLE without disease activity, in patients with other inflammatory diseases, and in controls, who did not undergo a renal biopsy. With a cutoff point of 13.5pg/mg Cr, this group presented with a sensitivity of 50% and a specificity of 90% in the diagnosis of renal activity for lupus. Unfortunately, the Schwartz study did not include patients with other glomerulopathies, and they analyzed the levels of uTWEAK in patients who had already received previous immunosuppressive treatment, which means that it is plausible that the levels of uTWEAK might have been modified.21 Another study23 performed in China, evaluated the levels of uTWEAK as a biomarker of renal lupus activity in 46 patients with SLE (12 without renal activity and 34 with LN); their results showed significantly higher urinary levels of TWEAK in patients with LN. However, this study, which was performed by Xuejing et al., only included patients with lupus and did not evaluate healthy controls or patients with other types of glomerulopathies. In a recently study Salem et al., observed that the patients with lupus nephritis have a higher uTWEAK levels in comparison with patients without renal activity, they found a sensitivity 100% and specificity of 66.67% as a biomarker of nephritis in SLE. The sensitivity and specificity of the uTWEAK level as a marker of active nephritis in SLE patients were 100% sensitivity and 80% specificity and area under the curve was 0.960 (p<0.0001), although in this study did not specified the previous treatment and the response to it.31
As a result, until now, it was not known how uTWEAK levels might affect patients with other types of glomerulopathies of possible autoimmune origin. We observed that the levels of uTWEAK in patients with non-lupus glomerulopathies were significantly lower than those observed in patients with active LN. However, when we compared the levels of urinary TWEAK in patients with other non-lupus glomerulopathies with the levels of urinary TWEAK in healthy volunteers and in patients with SLE without disease activity, significantly higher values were observed.
Similarly, it is possible that the increment in the levels of urinary TWEAK might reveal the immunological background of the activation of the tumor necrosis factor superfamily in patients with autoimmune glomerulopathies.28,29 Despite this, we consider that the values of uTWEAK in patients with glomerulopathies were significantly lower with respect to the values of TWEAK that were analyzed according to the cutoff point established through the ROC curve for the diagnosis of active lupus nephritis.
On the contrary, our results showed that the levels of uTWEAK were a specific characteristic of lupus nephritis activity and not a reflection of systemic activity (SLEDAI scores). Until now, clinical markers that have been available for the evaluation of patients with LN are included within the SLEDAI clinical index, which has established four parameters for renal affectation.4,30 A maximal SLEDAI score of 16 suggests disease activity, but one of the observations of the SLEDAI is that even in the absence of this score, a diagnosis of active LN cannot be ruled out because the SLEDAI scores reflect systemic lupus activity.27 Based on this previous finding, our results showed how SLEDAI scores above 3 (active lupus) and the values of uTWEAK permit the identification of lupus renal activity independently of systemic activity.
In relation to biochemical and immunological parameters, an inversely proportional correlation was observed between the levels of uTWEAK and the levels of C3 and C4 complement proteins. A directly proportional correlation was observed between the levels of TWEAK and the 24-h levels of proteinuria. However, a correlation was not observed between the levels of urea and creatinine, which is a finding similar to what has been previously reported in the literature, where biochemical markers demonstrate a moderate correlation with the presence of LN.21–23
As was mentioned above, renal affectation due to SLE is a severe complication that has a direct impact on patient survival.1–3 The diagnosis of lupus nephropathy is based on clinical features, biochemical parameters and immunological markers; however, no specific biomarker correctly defines renal activity for lupus. The results of our work show how urinary levels of TWEAK allow for the adequate classification of patients with active LN. This biomarker may be useful due to its high sensibility with respect to other established markers like a screening test. However, in contrast to what has been published in previous studies, our results do not show statistically significant correlations between the values of uTWEAK and the histological findings, at same, our levels were lower than other studies21,31 may be relate whit the histological and immunological change due previous treatment and in some cases the patients in others studies received more than one line of treatment this could change the levels of uTWEAK. As a result, renal biopsy should continue as the gold standard for the histological diagnosis of specific activity and chronicity in patients with lupus nephritis. However, from our perspective, we consider that additional clinical studies should be performed to define the possible role of urinary levels of TWEAK as a potential biomarker for the measurement of treatment response, the evaluation of possible relapses of renal activity, and for the evaluation of patients with lupus nephropathy who are seronegative for lupus. At the same time, the levels of uTWEAK should be considered a useful diagnostic biomarker in patients when a kidney biopsy is not indicated (e.g., pregnant women and patients with coagulation disorders). As a matter of act the urinary TWEAK levels with this cut-off point could be useful in diagnosis of lupus nephritis only in patients without previous treatment, is necessary determinate the modifications in uTWEAK levels due treatment.
In conclusion, we can establish that in Mexican patients who were recently diagnosed with SLE, the levels of uTWEAK were an appealing biomarker that showed higher sensitivity and specificity compared with those of the commonly used and previously described biomarkers.
Weakness of studyWe consider that the size of the sample is limited, so that the variations in the standard deviation can correspond to this. We observed a predominance of class IV+V so we could not establish the correlation with histological findings, we had not have patients with class V, in a previous study is noted that this histological pattern have the uTWEAK higher than other classes. We consider important establish the correlation between histological findings urinary TWEAK levels also plasma levels, in our study we found a different cut-off point, we believe this may be due a previous treatment; in order to improve the accuracy of urinary TWEAK levels as a diagnosis test is relevant determinate if there are modifications in the urinary levels of TWEAK secondary to treatment.
Authorship- 1.
Fabiola Reyes Martinez: participated in the conception and design of the study as well as the generation, collection, assembly, analysis and interpretation of the data.
- 2.
Monserrat Perez Navarro: participated in the design of the study, analysis and interpretation of the data, and the drafting and revision of the manuscript.
- 3.
Adrian Rodríguez Matías: participated in the conception and design of the study, analysis and interpretation of the data, and the drafting and revision of the manuscript.
- 4.
Virgilia Soto Abraham: participated in the analysis and interpretation of the renal biopsy, and the drafting and revision of the manuscript.
- 5.
Gabriela Gutierrez-Reyes: participated in the analysis and interpretation of urinary TWEAK levels, and the drafting and revision of the manuscript.
- 6.
Zaira Medina-Avila: participated in the analysis and interpretation of urinary TWEAK levels.
- 7.
Rafael Valdez-Ortiz: participated in the conception and design of the study as well as the drafting, revision and approval of the final version of the manuscript.
The authors have no conflicts of interest to disclose.
The authors express their gratitude to the Department of Experimental Medicine of the Hospital General de México, Dr. Eduardo Liceaga and Universidad Nacional Autónoma de México. They would also like to thank the Instituto Carlos Slim de la Salud for the scholarship awarded to Dr. Fabiola Reyes-Martínez, which allowed her to participate in this research project.