To the Editor:
Glomerular deposits of monoclonal immunoglobulins can arise as a condition secondary to a number of different entities, including AL amyloidosis, Randall type monoclonal immunoglobulin deposition disease (MIDD), type 1 cryoglobulinaemia, immunotactoid/fibrillar glomerulonephritis, and the most recently described, non-Randall type proliferative glomerulonephritis with monoclonal IgG glomerular deposits (MIgG PGN).1
Here we described the case of a patient with nephrotic syndrome and renal failure, whose examination led to the diagnosis of multiple myeloma and MIgG PGN.
Our patient was a 76-year old male who sought treatment due to renal failure and oedema, with two months evolution. The patient’s medical history only mentioned arterial hypertension. A physical examination revealed pitting oedema in the legs up to the knees and no other relevant findings. Complementary tests revealed: haemoglobin: 7.7g/dl; creatinine: 3.4mg/dl; albumin: 2.1g/dl; total cholesterol: 232mg/dl; IgG: 304mg/dl; IgA: 958mg/dl; IgM: 40mg/dl; C3: 79mg/dl; all other tests, including C4, rheumatoid factors, antinuclear antibodies, anti-DNA antibodies, anti-neutrophil cytoplasmic antibodies, anti-GBM, and cryoglobulin results were all normal/negative. Serology tests for hepatitis B and C virus and human immunodeficiency virus were all negative. Urine analysis: proteinuria: 11g/24h; urinary sediment: 6-12 leukocytes per field, 250 red blood cells per field, and negative urine culture. High resolution electrophoresis of urine and blood samples revealed a lambda IgA monoclonal component. A bone marrow core sample revealed a hypercellular medulla with 13% plasma cells of aberrant morphology, compatible with the diagnosis of lambda IgA type multiple myeloma. A chest x-ray, abdominal ultrasound, and computed axial tomography of the chest, abdomen, and pelvis all failed to produce relevant findings.
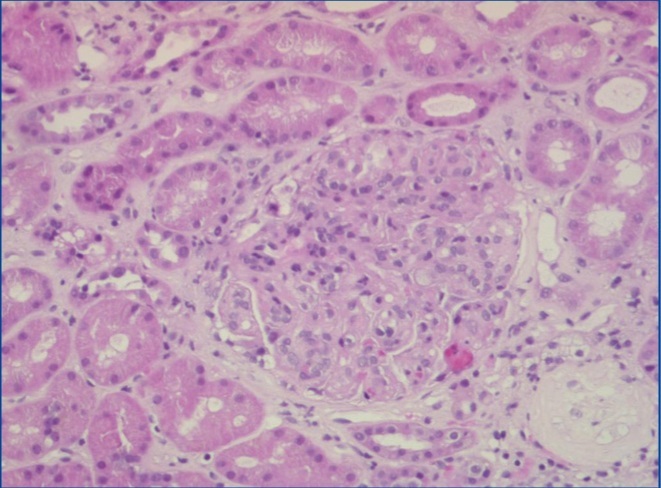
A percutaneous renal biopsy (23 glomeruli) revealed mesangial and endocapillary proliferation with splitting/thickening of the capillary walls (Figure 1); immunofluorescence revealed evidence of IgG, C3, C1q, and lambda chain deposits in the glomeruli studied; kappa chain stain test was negative; no relevant deposits were observed in the tubules; and Congo red stain was negative. In the electron microscope analysis, we observed fused foot processes, mesangial interpositioning, and subendothelial electrodense deposits; there were no fibrils or microtubules. These findings were compatible with the diagnosis of MIgG PGN. We treated the patient with dexamethasone and bortezomib. Three months later, the patient sought treatment for acute enteritis and bacteraemia from Escherichia coli, with deteriorated renal function that did not recover, and the patient was started on haemodialysis.
Nasr et al.2 considered MIgG PGN as an entity defined by glomerular deposition of monoclonal IgG (predominantly IgG3), along with a light chain isotype, absence of tubular deposits, and electron microscope findings similar to those from cases of immunocomplex glomerulonephritis; in addition, these authors defined this condition as involving an absence of clinical and laboratory evidence of cryoglobulinaemia. In a 37-patient study, the most common histological forms were membranoproliferative GN and endocapillary proliferative GN; nephrotic syndrome and renal failure were the most common forms of presentation. In 30% of cases, there was also a monoclonal component encountered in serum samples, but only one case of myeloma; 10 patients had hypocomplementaemia. In another study,3 membranous GN was the predominant form, and other haemopathies were encountered in addition to myeloma, such as chronic lymphatic leukaemia and non-Hodgkin’s lymphoma. Sethi et al.4 also described patients with membranoproliferative GN, associated with monoclonal gammapathy, with glomerular deposits of monoclonal IgG and IgM.
The differential diagnosis of MIgG PGN must take into account the aforementioned diseases, and especially Randall type MIDD (heavy/light chain deposition type).5 In this disease, the most typical glomerular involvement is nodular glomerulosclerosis, although membranoproliferative GN can also be observed, tubular deposits are practically a constant, and the electron microscope findings would be different. However, some authors include MIgG PGN within the spectrum of MIDD.6 For now, it is still not clear whether considering MIgG PGN as a distinct entity may produce therapeutic repercussions.
In our patient, the immunofluorescence result of a unique light chain isotype suggests a type of nephropathy from monoclonal immunoglobulin deposition; the absence of tubular deposits and electron microscope findings were in favour of the diagnosis of MIgG PGN. There was a discrepancy between the monoclonal peak encountered in serum samples (lambda IgA) and in deposits (lambda IgG), which has already been described in another case of MIgG PGN,7 and which would be due to a rapid tissue precipitation of the IgG component, or concentrations in serum samples that are below the detectable threshold.
To conclude, MIgG PGN must be considered as a possible diagnosis, among others, in patients with glomerular deposits of monoclonal immunoglobulin.
Conflicts of interest
The authors state that they have no potential conflicts of interest related to the content of this article.
Figure 1. Light microscope image. HE x10. Glomerulus with mesangial and endocapillary proliferation and thickening of capillary walls