Obesity is associated with the early onset of glomerulomegaly, hemodynamic changes of a hyperfiltering kidney, and increased albuminuria, which are potentially reversible with weight loss. However, pathologic lesions of focal segmental glomerulosclerosis develop in experimental models of sustained obesity, and are observed in morbidly obese humans presenting with massive proteinuria. In addition, several observational, cross sectional and longitudinal studies document that obesity is as an independent risk factor for the onset, aggravated course, and poor outcomes of chronic kidney disease, even after adjustment for confounding co-morbidities including metabolic syndrome, diabetes and hypertension, the major causes of chronic kidney disease. Early dietary intervention to reduce weight, and where necessary bariatric surgery, should be considered in the management of overweight and obese chronic kidney disease (CKD) patients.
La obesidad está relacionada con la aparición temprana de glomerulomegalia, las alteraciones hemodinámicas del riñón hiperfiltrante y el aumento de la albuminuria, síntomas que son reversibles con una pérdida de peso. Por el contrario, las lesiones patológicas de la glomeruloesclerosis focal y segmentaria se desarrollan en modelos experimentales de obesidad mantenida y se observan en pacientes con obesidad mórbida que presentan proteinuria masiva. Además, diferentes estudios observacionales, transversales y longitudinales han demostrado que la obesidad supone un factor de riesgo independiente de la aparición, el empeoramiento, y la escasa respuesta al tratamiento de la enfermedad renal crónica (ERC), incluso después de ajustar por variables de confusión, incluido el síndrome metabólico, la diabetes y la hipertensión, las principales causas de la enfermedad renal crónica. En los pacientes con enfermedad renal crónica que presentan sobrepeso y obesidad debe considerarse la instauración precoz de una dieta para reducir peso y la cirugía bariátrica, en caso de ser necesaria.
INTRODUCTION
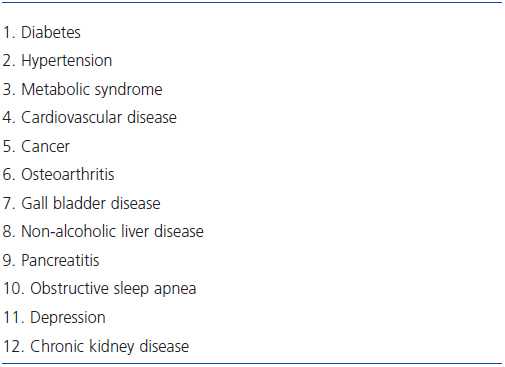
Throughout most of human history corpulence has been considered a sign of good health and being fat an advantage. The potential of harmful effects of excess body weight were appreciated in the 19th century, but it is only in the early decades of the 20th century that the complications and increased morbidity and mortality of obesity began to be documented1. Since then, the accrued evidence clearly indicates that fat cells provide not merely energy storage but that components of excess adipose tissue function as an endocrine organ with multiple detrimental health consequences, and that obesity frequently contributes to the pathogenesis, complicates the course, and increases the risk of several diseases including diabetes, hypertension, cardiovascular disease, metabolic syndrome, certain malignancies, and kidney disease (Table 1)2,3. The importance and urgency of identifying the detrimental consequences of obesity has been the dramatic increase in its incidence and prevalence after the Second World War. Population surveys indicate that two thirds of United States adults are overweight and one third obese4,5. Similar trends documented in other parts of the world have led to the identification of obesity as a worldwide public health problem of epidemic proportions with increasing incidence, high costs, and poor outcomes1-6.
The first renal complication to be associated with obesity was renal cell carcinoma7. The effects of obesity on kidney function and disease have since been identified and become a subject of increased study and concern8-12. Actually, an effect of obesity on albuminuria and blood pressure in kidney disease was reported as early as 192313, when actuarial data first began to identify overweight as a risk factor for mortality, but were forgotten or neglected when cardiovascular mortality emerged as the principal cause of obesity-related mortality1. Based on a meta-analysis, it has been estimated that the presence of kidney disease is related to overweight (BMI = 25-29.9) and obesity (BMI ≥30)14. An association between obesity and kidney disease could be made in 24.2% of males and 33.9% of females in the U.S., and in industrialized countries in 13.8% of men and 24.9% of women. Furthermore, obesity was shown to adversely affected the progressive loss of kidney function among those with chronic kidney disease (CKD)14. Regrettably, the detrimental renal effects of obesity remain unrecognized and are not included in major reviews of obesity3.
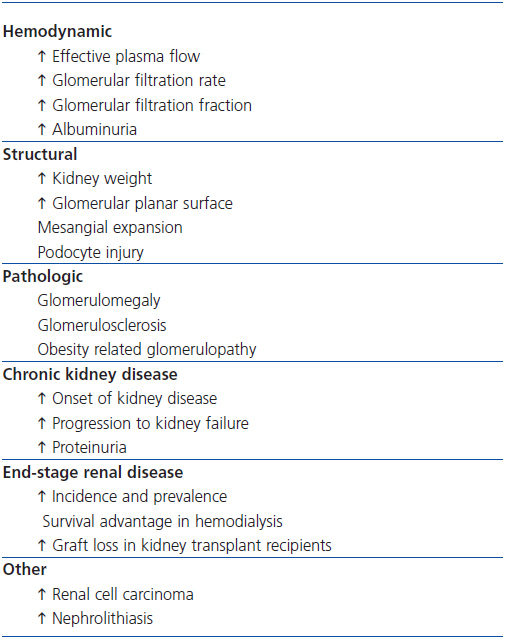
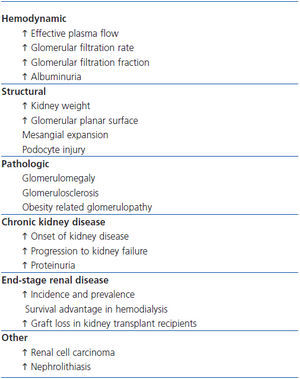
This article reports the effects of obesity on the normal and diseased kidney (Table 2), considers the factors implicated in their pathogenesis, and presents an approach to their management.
EFFECTS OF OBESITY ON RENAL HEMODYNAMICS
Experimental studies in genetically obese rats and force fed dogs show an early onset of hemodynamic changes in kidney function characterized by an increase in glomerular filtration rate (GFR) and effective plasma blood flow, accompanied by variable increments in filtration fraction and albumin excretion15,16. These early changes are reversible as shown in studies of hyperphagic obese Zucker rats in which, early (at 6 or 12 weeks of age) but not late (after 26 weeks of age), food restriction was associated with reversal of glomerular hyperfiltration and albuminuria17.
Cross sectional and longitudinal clinical studies confirm these hemodynamic changes characteristic of a hyperfiltering glomerulus in humans, whose first clinical manifestation of renal injury appears to be increased albuminuria18,19. Compared to lean subjects, in severely obese individuals the GFR and renal plasma flow were shown to be higher by 51 and 31 percent, respectively20. Glomerular macromolecular sieving studies in these individuals indicate that a principal reason for these hemodynamic effects and attendant increase in glomerular filtration fraction is due to afferent arteriolar dilatation. An added contributory role of efferent arteriolar vasoconstriction on the increased filtration fraction, due to stimulation of the renin-angiotensin system, has been documented also and is supported by the salutary effect of angiotensin receptor blocking drugs on the albuminuria and renal hemodynamics in experimental and clinical studies of obesity21,22. Evidence also exists for a role of obesity-induced increase in renal sympathetic tone that could contribute and aggravate these hemodynamic changes23,24.
A significant correlation between increased urinary albumin excretion and body weight has been shown in both non-diabetic and diabetic overweight individuals19,25-27.The effect of obesity on proteinuria is not bimodal, but a continuum that is directly related to increasing body mass index (BMI). In a retrospective analysis of the database of a population study on the impact of microalbuminuria on renal and cardiovascular risk, the prevalence of microalbuminuria (30-300 mg/d) in men increased from 9.5% in those with normal body weight (BMI <25) to 18.3% in those who were over-weight, and to 29.3% in those who were obese; in women, the respective percentages were 6.6%, 9.2%, and 16.0%26.
The hemodynamic effects of overweight on kidney function and albuminuria are magnified in the presence of hypertension, which itself is a clinical complication of obesity10,19,28. A similar amplifier effect of obesity has been reported in overweight diabetics27. In a cross-sectional study of risk factors for microalbuminuria among African Americans with newly diagnosed type 2 diabetes, 23.4% of whom had microalbuminuria, the urine albumin to creatinine ratio was independently associated with BMI27. Importantly, moderate weight reduction (4.1%) in overweight diabetics who were proteinuric decreased their proteinuria by about 30%29 There is now convincing evidence of a salutary effect of weight reduction, by bariatric surgery or dietary caloric restriction, on the proteinuria of obese individuals30,31.
In evaluating kidney function in obese individuals, it is important to note that currently available methods of estimated GFR (eGFR) were derived in lean individuals; both the Cockcroft-Gault formula and the MDRD equation are less accurate in malnourished as well as obese individuals. The Cockcroft-Gault formula grossly overestimates eGFR and should not be used in obese individuals. The MDRD equation appears to be more dependable in obesity, but also tends to err by 10 ml/min/1.73 m2 or more in obese cases32,33.
EFFECTS OF OBESITY ON RENAL MORPHOLOGY AND PATHOLOGY
In force-fed dog studies, the hemodynamic changes of obesity were associated with an increase in kidney weight of about 40%10,15. This was accompanied by an increase of glomerular size together with podocyte injury and expansion of the mesangium, and in sustained obesity resulted in mesangial sclerosis15. As with the hemodynamic changes, these early structural changes of obesity were prevented by dietary restriction in hyperphagic Zucker rats16.
In humans, despite the occurrence of glomerulomegaly, hyperfiltration, and albuminuria most obese individuals do not develop glomerulosclerosis34. In a study comparing kidney biopsies from obese to lean living kidney donors the glomerular planar surface area was significantly greater in those who were obese but showed no evidence of glomerulosclerosis18. However, cases of glomerulomegaly, focal segmental glomerulosclerosis, proteinuria, and decreased kidney function do occur in obesity, a clinicopathothological entity that has been termed obesity-related glomerulopathy35. The increased prevalence of obesity appears to be reflected in an increased incidence of obesity-related glomerulopathy, as shown in a retrospective analysis of kidney biopsies at one center that revealed a 10-fold increase in its occurrence over the 15 year period of the study36. The occurrence of glomerulosclerosis only in selected cases of obesity may be related to genetic predisposition, the presence of other co-existent risk factors, and the severity and duration of obesity.
Clinically, obesity-related glomerulopathy is associated with proteinuria and was first described in 1974 in four morbidly obese patients (BMI >50 kg/m2) who presented with nephrotic syndrome, none of whom were diabetic and only two were hypertensive37. Kidney biopsies on two of them revealed focal and segmental glomerulosclerosis. In all four cases the proteinuria decreased during dietary-induced weight loss. Nephrotic range proteinuria has been reported also in severe obesity with normal kidney biopsy38. Reduction of nephrotic range proteinuria in obese diabetics occurs with weight loss after gastric bypass surgery39,40. In morbidly obese individuals who develop non-nephrotic range albuminuria, the histologic changes appear to precede the onset of microalbuminuria, and the onset of proteinuria precedes the decline in GFR by several years41.
The long term prognosis of obesity-related glomerulopathy is poor, but unlike idiopathic focal segmental sclerosis, in obesity-related glomerulopathy the incidence of nephrotic range proteinuria is lower, the serum albumin higher, the serum cholesterol lower, the edema less severe, and the progression to end-stage renal disease slower34,42.
EFFECT OF OBESITY ON PROGRESSION OF KIDNEY DISEASE
In population based epidemiologic studies, obesity has been shown to be associated with new onset of CKD and increased rate of progression to kidney failure in individuals with existing primary kidney disease8-12. In a study of predictors of new onset kidney disease, in a cohort of 2585 patients followed for 20 years, the odds ratio for new onset of chronic kidney disease was 1.23 per one standard increase in BMI43. Increased BMI has also been shown to increase the risk of progression of existing kidney disease, adjusted for confounders including diabetes and hypertension. Obese individuals with CKD have a higher rate of decline in glomerular filtration rate and progress faster to end-stage renal disease (ESRD)44. In a large population study evaluating the risk for ESRD over a span of 20 years, increased BMI was an independent risk factor for progression to ESRD in obese individuals compared to those with normal body weight45. In another population-based case controlled study, a BMI of over 25 at age 20 was associated with a threefold increased risk for developing new onset of kidney disease, even after correction for hypertension and diabetes46. The coexistence of diabetes and obesity in this study doubled the risk for new onset of kidney disease.
Direct evidence for a detrimental effect of obesity on kidney disease comes from the study of specific diseases such as IgA nephritis, where excessive body weight (BMI ≥25) at the time of kidney biopsy was shown to be associated with the severity of the detected pathologic lesions, the subsequent rate of loss of kidney function, and to be an independent risk factor for progression to ESRD41,47. Similar results have been observed on the onset of proteinuria and progressive loss of kidney function after unilateral nephrectomy in obese individuals48. At the 20-year follow up of this study, most of the normal non-obese individuals had normal kidney function as compared to only about 40% of the expected GFR for age in the obese subjects. These observations are more dramatically evident in kidney transplant recipients. In an analysis of a large registry database (51927 kidney transplant recipients), it was shown that the relative risk of graft loss, patient death, and cardiovascular mortality increased49 by 20-40% at increasing BMIs of over 30 kg/m2. As a result, a BMI of over 35 kg/m2 has come to be considered a contraindication to kidney transplantation in some centers.
The detrimental effect of overweight on the course of CKD is supported by several epidemiologic studies that show a higher prevalence and increasing incidence of obesity in patients with ESRD being initiated on dialysis50. In a study of over 300,000 subjects, the presence of obesity was shown to increase the relative risk of ESRD, adjusted for confounding factors45. Once again, this relationship was not related to obesity bi-modally, but showed an incremental continuum related to increased BMI that was statistically significant at BMI values of >25. Compared to lean subjects, the relative risk of ESRD was 3.57 for those with a BMI of 30-34.9, 6.12 for those with a BMI of 35-39.9, and 7.07 for those with morbid obesity (BMI >40)45.
EFFECT OF OBESITY IN CHRONIC AND ACUTE DIALYSIS PATIENTS
Contrary to the convincing evidence of a detrimental effect of obesity on the onset, course, and outcomes of CKD, obesity in dialysis patients appears to provide a survival advantage, an effect that has been dubbed “reverse epidemiology”51. This striking disparity with the usual detrimental effects of obesity on survival in CKD may be due to the relatively higher mortality of chronic kidney disease patients during the progression of their disease with only those having inherent survival advantages progressing to ESRD, and the fact that the initial reported analysis compared variable survival data (10 years for normal, 4 years in dialyzed patients). In a study in which subjects were followed for a comparable period, obesity was shown actually to increase mortality in dialysis patients52.
Another reason for an advantage of obesity in dialyzed patients may be teleological. The genetic origins of obesity rooted in the survival advantage it provided at times of famine may be operative in ESRD patients on dialysis. Maintenance hemodialysis is essentially a catabolic state similar to malnutrition in which obesity may convey some survival advantage to dialysis patients, much like it did to their ancestral hunters-gatherers at times of food scarcity1,53. The survival advantage proffered by obesity is not limited to dialyzed patients but is also observed in other chronic diseases such as congestive heart failure, liver cirrhosis, and obstructive pulmonary disease3,6,54.
Relevant to this issue is the finding that obesity also confers survival advantage in intensive care unit (ICU) patients requiring renal replacement therapy54. In the case of CKD, obesity was an independent risk factor for developing acute kidney injury in the ICU, but conveyed improved survival in those requiring dialysis.
OTHER EFFECTS OF OBESITY ON THE KIDNEY
Obesity is associated with an increased risk for renal cell carcinoma7. Past cautiousness in considering this as the only renal complication of obesity likely reflects the fact that the renal complications of obesity were initially attributed to be secondary to the common association of obesity with hypertension and diabetes, the two most common causes of chronic kidney disease6. An improper caution refuted by the data reviewed here, but an error that regrettably continues to be perpetuated in the current literature3.
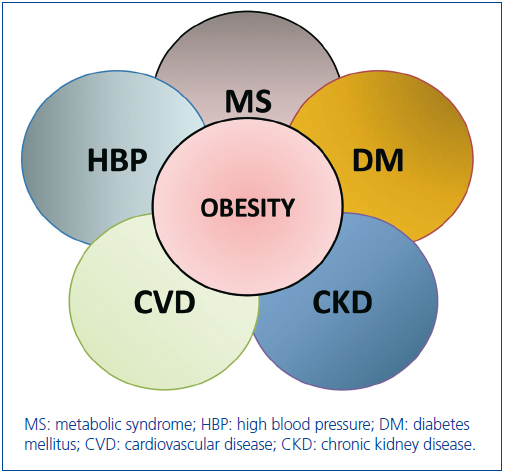
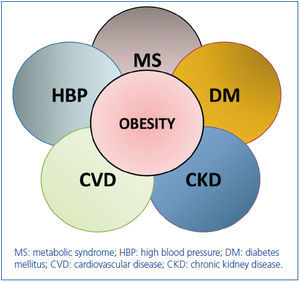
Actually, the relationships between obesity, hypertension, diabetes, cardiovascular disease, metabolic syndrome and CKD can be viewed as the clinical intersection of a cluster of diseases that are associated with kidney disease (Figure 1). The coexistence of obesity in any of these diseases (diabetes, hypertension, metabolic syndrome, cardiovascular disease) magnifies the risk of onset of kidney disease and its progression to ESRD. Conversely, the coexistence of obesity and chronic kidney disease in any of those other diseases increases their risk of morbidity and mortality6.
OBESITY, DIABETES, AND THE KIDNEY
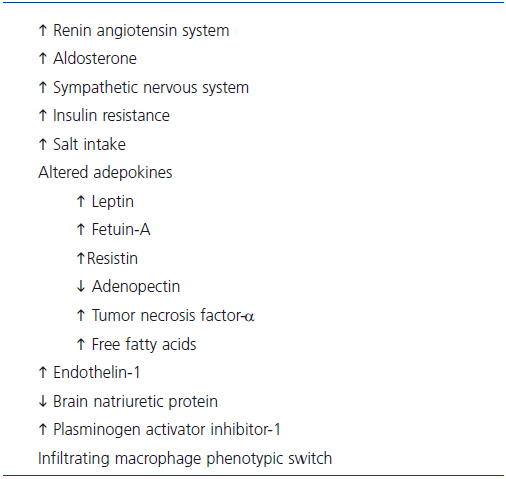
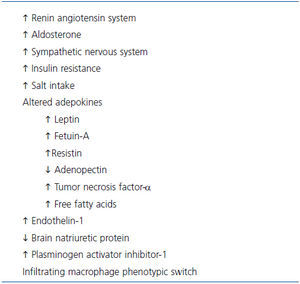
Whereas thermodynamic studies clearly establish fat deposition as a consequence of imbalance between the energy derived from ingested food and that of energy expended in the course of daily activities, obesity is in fact a multifactorial disease in which the adipose tissue rather than just being a site for excess energy storage actually functions as an endocrine and exocrine organ with neurohumoral and vasoactive effects that are implicated in the genesis of obesity-related organ damage including the kidney (Table 3). In addition to angiotensinogen and renin, adipose tissue produces several cytokines, growth factors, and bioactive adepokines that have been implicated in or associated with kidney injury13,53-63.
Among the commonly implicated mechanisms of organ damage is that of insulin resistance that characterizes the obese and type II diabetics58, and may account for the hemodynamic disorders of obesity-related hyperfiltration, glomerulomegaly, and albuminuria that are also the characteristics of early diabetic nephropathy. Although an association between microalbuminuria and BMI is a feature of non-diabetic obese subjects, in clinical studies of diabetics it is rather difficult to separate the effects of increased BMI alone from its concomitant effects on glycemic control27. However, the lesions of obesity-related glomerulopathy are distinctly different from those of diabetic nephropathy6. The characteristic lesions of nodular sclerosis and membranous thickening of diabetic nephropathy are not observed in the classic lesions of obesity-related glomerulopathy, which is one of focal segmental glomerlosclerosis. In a study comparing the kidney biopsies of microalbuminuric diabetic subjects, there was no difference in glomerular pathology of obese compared to lean diabetics who were biopsied64. The severe hyperglycemia and advance glycated end products (AGE) in diabetes have been incriminated for these structural differences, but remain to be proven. Thus, whereas obesity per se results in distinct hemodynamic, structural, and pathologic changes in the kidney, in addition it is an independent amplifier of the risk associated with CKD in general and with diabetic nephropathy, in particular (Figure 1).
By the same token, whereas the lesions of obesity-related glomerulopathy are similar to those of idiopathic focal segmental glomerulonephritis, there are clinical differences apart from the glomerulomegaly of obesity that distinguishes them from each other6,34. Viewed in an evolutionary framework, the millennia of human evolution during which the genetic advantages of obesity changed it from one of survival advantage to a disease after food became easily available, was accompanied by the evolution of genetically diverse populations, who are divergent in their genetic susceptibility to the consequences of diseases in general and of obesity-related renal disease in particular. This may account, at least in part, why the renal morphologic complications of obesity are encountered clinically in some but not all obese individuals, whereas its detrimental hemodynamic effects are variably expressed, in most of those with primary forms of CKD, including diabetic nephropathy. Coupled with environmental factors and other acquired life style changes (smoking, exercise, type of food) this may account for the variations in obesity-related complications in general and that of obesity-related glomerulosclerosis in particular. Obviously, there remain many gaps in our current knowledge of these effects that remain to be explored and explained.
CONCLUSION
In summary, a growing body of evidence that is convincing in balance indicates specific hemodynamic, structural, and functional changes of the kidney in obesity, and the onset of obesity-related glomerulosclerosis in some of them. In addition, the presence of obesity amplifies the risk for and outcomes of chronic kidney disease in diabetes, hypertension, metabolic syndrome, primary kidney diseases, and cardiovascular disease (Figure 1). Given the evidence for the potential reversibility of these detrimental obesity-related hemodynamic effects and the proteinuria of obesity on progression of kidney diseases and their outcomes, weight reduction (dietary or bariatric surgery) should be considered a component of the therapeutic regimen of all overweight individuals with CKD30,31.
Apart from caloric restriction, given the tendency to salt retention of obesity salt restriction must be part of their dietary management. In addition, because of the acid load of the high protein diet consumed by obese individuals consideration should be given to the use of bicarbonate supplementation in those with reduced kidney function65.
Also, given the increased activity of the renin-angiotensin system in obesity angiotensin converting enzyme inhibitors and angiotensin receptor blocking agents should be part of their therapeutic regimen, especially in the presence of proteinuria and hyperension6,12,34.
Table 1. Co-morbidities associated with overweight and obesity
Table 2. Effects of overweight and obesity on the kidney
Table 3. Factors implicated in the pathogenesis of CKD in obesity
Figure 1. The cluster of co-morbidities associated with and aggravated by obesity. Where there is clinical intersection of a given circle with that of obesity, the co-existence of obesity emerges as a risk multiplier of the outcomes of that disorder. The areas wher