High serum gamma-glutamyl transferase (GGT) levels are associated with increased mortality in the general population. However, this association has scarcely been investigated in patients with chronic kidney disease (CKD).
This study aims to investigate the clinical characteristics of CKD patients with abnormally elevated serum GGT, and its value for predicting mortality.
Material and methodsRetrospective observational study in a population cohort of adults with stage 4–5 CKD not yet on dialysis. Demographic, clinical, and biochemical parameters of prognostic interest were recorded and used to characterise CKD patients with high levels of GGT (>36IU/L). Cox proportional hazard regression models were used to analyse the influence of baseline serum GGT and alkaline phosphatase (ALP) levels on mortality for whatever reason.
ResultsThe study group consisted of 909 patients (mean age 65±15 years). Abnormally elevated GGT or ALP levels at baseline were observed in 209 (23%) and 172 (19%) patients, respectively, and concomitant elevations of GGT and ALP in 68 (7%). High GGT levels were associated with higher comorbidity burden, and a biochemical profile characterised by higher serum concentration of uric acid, triglycerides, alanine aminotransferase, ferritin, and C-reactive.
During the study period, 365 patients (40%) died (median survival time=74 months). In adjusted Cox regression models, high levels of GGT (hazard ratio [HR]=1.39; CI 95%: 1.09–1.78, P=0.009) and ALP (HR=1.31; CI 95%: 1.02–1.68, P=0.038) were independently associated with mortality.
ConclusionHigh serum levels of GGT are independent predictors of mortality in CKD patients.
Los niveles séricos elevados de gamma-glutamil transferasa (GGT) se asocian con una mayor mortalidad en la población general, pero es desconocido si esta asociación también ocurre en pacientes con enfermedad renal crónica (ERC).
Los objetivos de este estudio fueron investigar las características clínicas de los pacientes con ERC y elevación de los niveles séricos de GGT, así como el valour de predicción de esta enzima sobre la mortalidad.
Material y métodosEstudio retrospectivo de observación en una cohorte de pacientes con ERC estadios 4-5 prediálisis. Se recogieron los parámetros demográficos, clínicos y bioquímicos de interés pronóstico y se utilizaron para caracterizar a los pacientes con elevación de GGT (>36 UI/l). Mediante regresión de Cox se analizó la asociación de los valores basales de GGT y fosfatasa alcalina (FA) sobre la mortalidad por cualquier causa.
ResultadosSe incluyó a 909 pacientes (edad media 65±15 años). Se observaron niveles elevados de GGT o FA en 209 (23%) y 172 (19%) pacientes, respectivamente, y elevación simultánea en 68 (7%) pacientes. Niveles elevados de GGT se asociaron con mayor comorbilidad y un perfil bioquímico con concentraciones más elevadas de ácido úrico, triglicéridos, transaminasa glutámico-pirúvica, ferritina, y proteína C reactiva.
Durante el seguimiento fallecieron 365 pacientes (40%). Niveles elevados de GGT (hazard ratio [HR]=1,39; IC 95%: 1,09-1,78; p=0,009), o de FA (HR=1,31; IC 95%: 1,02-1,68; p=0,038) se asociaron de forma independiente con la mortalidad.
ConclusionesNiveles séricos elevados de GGT o de FA son predictores independientes de mortalidad en pacientes con ERC.
Chronic kidney disease (CKD) is associated with high mortality, particularly of cardiovascular-related origin. However, the limited association between mortality and traditional cardiovascular risk factors in these patients is paradoxical. Only some markers related to inflammation or nutrition (e.g. C-reactive protein and serum albumin) have been shown to be consistent predictors of mortality in CKD.1
In recent years some observational studies have shown an association between elevated total serum alkaline phosphatase (ALP) concentrations and mortality in patients with CKD2–5; however, the biological and clinical significance of this finding remains unknown.
Gamma-glutamyl transferase (GGT) is an enzyme present in serum and on the outer surface of cells from different organs such as the liver, pancreas, intestine, lungs and kidneys.6 Serum GGT is not only a traditional marker of alcohol consumption and hepatobiliary diseases, but several studies have also shown an association between elevated serum GGT levels and cardiovascular disease, diabetes mellitus, hypertension and metabolic syndrome.7
GGT levels is a predictor of mortality in the general population; in fact, this enzyme has recently been included as one of a set of biochemical parameters that predict mortality.8 Furthermore, serum GGT levels could help to interpret high ALP values of unclear origin.
There are only few studies that have analysed the clinical and prognostic significance of serum GGT in CKD.9,10 The aim of this study was to investigate the clinical characteristics of CKD patients with abnormally elevated serum GGT levels and their interaction with ALP, and to determine the value of this parameter as a predictor of mortality.
Materials and methodsThis is a retrospective, observational study conducted on a cohort of adult patients being followed in the low clearance (advanced CKD) outpatients clinic of the Nephrology department of the Hospital Infanta Cristina, Badajoz, Spain, from January 2002 to October 2013. The study included all pre-dialysis patients aged >18 years with an estimated glomerular filtration rate (eGFR) <30mL/min/1.73m2. Patients with CKD due to renal transplant dysfunction were not included.
Demographic data and clinical parameters such as age, sex, body mass index (BMI), comorbidities and medication were recorded upon inclusion.
Comorbidity was evaluated using the Davies score,11 and patients were classified according to the aggregate of their different comorbidities: no comorbidities, mild-moderate (1 or 2 comorbid processes), or severe (3 or more comorbid processes). The main comorbidities recorded were: diabetes mellitus, heart failure, coronary artery disease, cerebrovascular or peripheral vascular disease, chronic obstructive pulmonary disease, cancer and chronic inflammatory processes.
In the present study, the term “liver disease” not only included patients diagnosed with chronic liver diseases, cirrhosis or liver transplants, but also patients with active alcoholism, or active hepatotropic virus infection (B or C). Non-alcoholic steatohepatitis (fatty liver disease) was a common ultrasound finding among these patients, although this disease was not included in the list of liver diseases.
Patients were followed up regularly from the time of inclusion in the study until their death, renal transplant, loss to follow-up or the end of the data collection period (31 December 2014).
Given the retrospective design of the study, individual informed consent was not obtained.
Biochemical measurementsBeginning in January 2002, serum GGT and total ALP were added to the routine biochemistry parameters measures in patients from our advanced CKD clinic.
GGT levels were determined using an autoanalyser (Advia Chemistry, Siemens Healthcare Diagnostics, New York, USA) by kinetic colorimetric method with gamma-glutamyl-3-carboxy-4-nitroanilide and glycylglycine. The coefficient of variation is less than 4.5% and the reference range 0–36U/L.
Serum ALP levels were also measured by autoanalyser (Advia Chemistry), with the ALPI method, which quantifies the Alkalinine Phosphatase activity by measuring the transphosphorylation of p-nitrophenylphosphate to p-nitrophenol in the presence of 2-amino-2-methyl-1-propanol. The coefficient of variation was less than 4% and the reference range was 45–120U/L.
To characterise patients with abnormally elevated serum GGT or ALP, baseline haematological and biochemical parameters (shown in Table 1) were also included in the study. The biochemical parameters were determined using conventional laboratory methods (Advia Chemistry, New York, USA). Parathyroid hormone (molecule 7-84) (PTH) levels were determined by automated chemiluminescent immunoassay (DiaSorin, Italy), serum albumin concentrations using the bromocresol green method, and high-sensitivity C-reactive protein by solid-phase chemiluminescent enzyme immunoassay (Immulite autoanalyser, Diagnostic Product Corporation, New York, USA).
Clinical and biochemical characteristics of the patient set and sub-groups according to abnormally high GGT or ALP values.
| Total | Normal GGT and ALP | High GGT | High ALP | Simultaneously high GGT and ALP | P* | |
|---|---|---|---|---|---|---|
| N patients | 909 | 596 | 141 | 104 | 68 | |
| Age (years) | 65 (15) | 66 (15) | 63 (13) | 66 (17) | 66 (14) | 0.234 |
| Sex, male/female | 485/424 | 316/280 | 81/60 | 46/58 | 42/26 | 0.094 |
| Comorbidity index % | ||||||
| None | 31 | 37 | 21 | 29 | 9 | |
| Mild-moderate | 52 | 48 | 63 | 59 | 54 | <0.001 |
| Severe | 17 | 15 | 16 | 12 | 37 | |
| Active smokers, % | 15 | 14 | 21 | 10 | 25 | 0.011 |
| Diabetes mellitus, % | 39 | 35 | 46 | 41 | 50 | 0.013 |
| Liver disease, % | 3 | 2 | 6 | 2 | 10 | <0.001 |
| Ischaemic heart disease, % | 16 | 14 | 10 | 16 | 38 | <0.001 |
| CNS or peripheral ischaemia, % | 21 | 19 | 18 | 23 | 34 | 0.031 |
| Heart failure, % | 18 | 16 | 21 | 14 | 37 | <0.001 |
| BMI, kg/m2 | 29.4 (6) | 29.2 (5.8) | 29.4 (6) | 30.6 (6.7) | 29.4 (5.9) | 0.176 |
| eGFR, mL/min/1.73 m2 | 14.7 (5.1) | 14.8 (5.1) | 14.6 (4.6) | 14.1 (4.4) | 15.6 (6.2) | 0.234 |
| Haemoglobin (g/dl) | 11.4 (1.7) | 11.4 (1.7) | 11.1 (1.7) | 11.4 (1.5) | 11.6 (1.8) | 0.205 |
| Serum uric acid, mg/dL | 7.6 (1.9) | 7.5 (1.9) | 7.9 (2.1) | 7.5 (1.7) | 8.4 (2.4)a | <0.001 |
| Total cholesterol, mg/dL | 186 (49) | 187 (48) | 191 (56) | 179 (42) | 182 (50) | 0.226 |
| Triglycerides, mg/dL | 147 (96) | 140 (76) | 167 (133)b | 140 (70) | 171 (163) | 0.003 |
| Serum albumin, g/dL | 3.9 (0.5) | 3.9 (0.5) | 3.8 (0.5) | 3.9 (0.5) | 3.8 (0.5) | 0.151 |
| Serum total calcium, mg/dL | 9.1 (0.8) | 9.2 (0.9) | 9.2 (0.7) | 9 (0.9) | 8.9 (0.8) | 0.049 |
| Serum phosphorus, mg/dL | 4.7 (1.1) | 4.7 (1) | 4.7 (0.9) | 4.6 (1.2) | 4.6 (1.2) | 0.851 |
| Serum bicarbonate, mEq/L | 21.4 (3.9) | 21.6 (3.8) | 21.2 (3.7) | 20.5 (4.4) | 22.1 (4.5) | 0.026 |
| PTH, pg/mL | 267 (220) | 241 (187) | 234 (184) | 448 (337)c | 281 (195) | <0.001 |
| ALT, IU/L | 18 (16) | 16 (8) | 25 (23)d | 15 (6) | 30 (39)d | <0.001 |
| GGT, IU/L | 36 (63) | 18 (8) | 69 (39)d | 19 (8) | 142 (181)d | <0.001 |
| ALP, IU/L | 99 (52) | 79 (20) | 89 (21) | 163 (67)e | 187 (88)e | <0.001 |
| Serum ferritin, ng/mLg | 173 (247) | 148 (179) | 284 (361)d | 112 (118) | 271 (465)d | <0.001 |
| C-reactive protein, mg/L | 9.9 (17.3) | 8.5 (15.6) | 14.6 (22.2)f | 6.9 (9.4) | 17.8 (24.2)f | <0.001 |
| Proteinuria, mg/g creatinine | 2462(2787) | 2227(2632) | 2726(3035) | 3023(3314) | 3109(2473) | 0.004 |
| Renin-angiotensin inhibitors, % | 79 | 80 | 78 | 78 | 72 | 0.502 |
| Beta-blockers, % | 26 | 25 | 30 | 22 | 35 | 0.140 |
| Diuretics, % | 60 | 59 | 62 | 60 | 65 | 0.829 |
| Statins, % | 53 | 54 | 53 | 55 | 47 | 0.762 |
| Alopurinol, % | 22 | 22 | 25 | 26 | 13 | 0.200 |
| Vitamin D analogues, % | 10 | 10 | 7 | 15 | 9 | 0.201 |
| Antiplatelet agents, % | 34 | 33 | 28 | 41 | 38 | 0.124 |
The data are presented as a mean (standard deviation), unless otherwise specified.
The MDRD-4 formula was used to estimate the glomerular filtration rate (eGFR).12
Serum ferritin was the only parameter with missing values. The mean of the available values was included in the descriptive analysis, but the missing values were not computed, and this variable was not included as a covariable in the survival analysis.
Outcome variableThe outcome variable was death due to any cause. Causes of death were classified into 5 groups: sudden death, cardiovascular, infectious, malignancy and other causes.
Statistical analysisParametric or non-parametric tests were used for the descriptive comparison of the continuous variables, depending on their characteristics; and, the Chi-square test was used for the categorical variables. The Pearson's test was used for the bivariate correlation analysis.
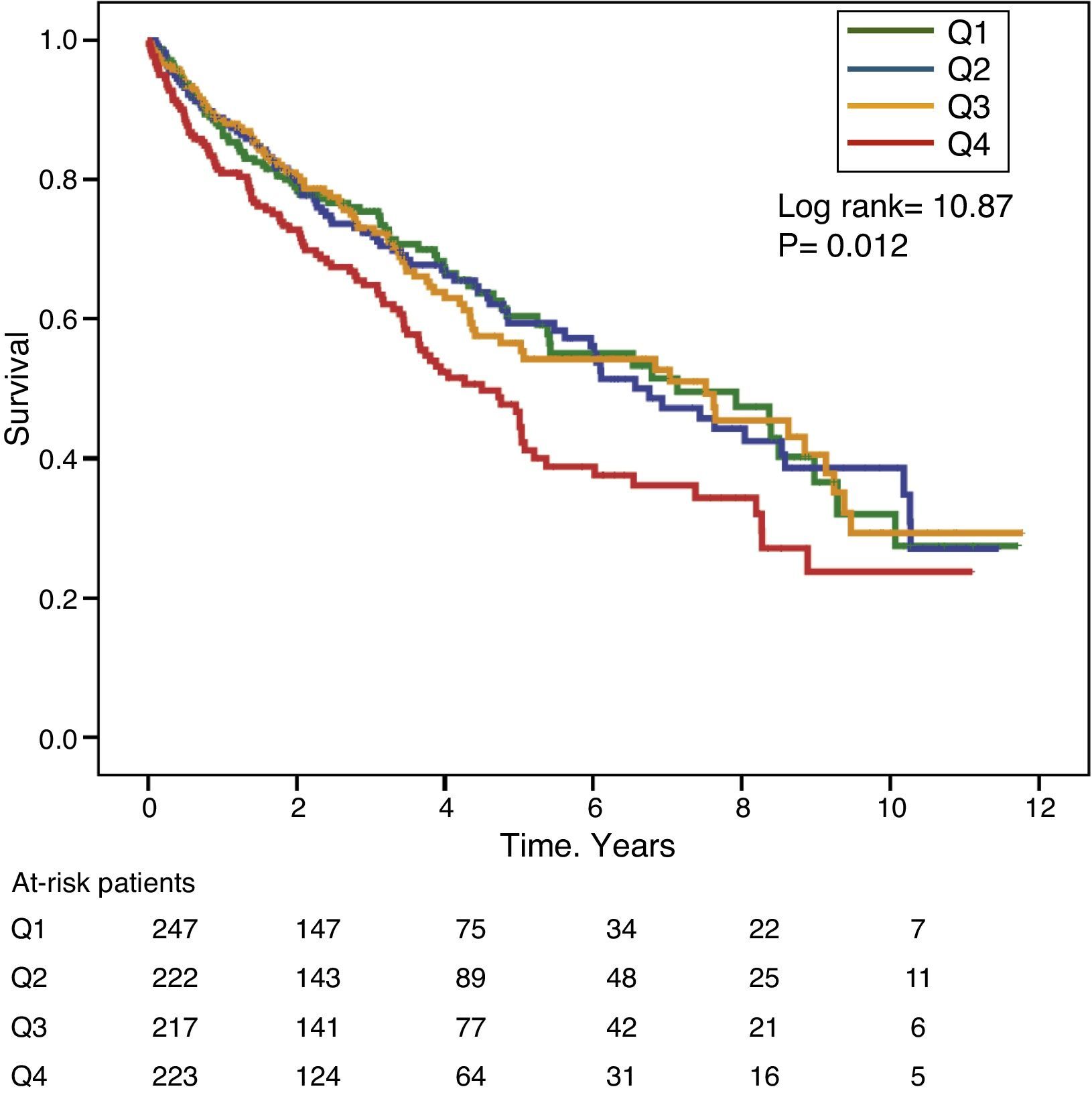
Differences in survival between patients according to the GGT or ALP quartiles were analysed using Kaplan–Meier curves, and compared using the Mantel–Haenszel log-rank test.
Multivariate Cox proportional hazard models were used to analyse the effect of the baseline GGT and ALP values on time to all-cause mortality.
Since the baseline GGT and ALP levels presented a positively-biased distribution, the values were logarithmically transformed for inclusion as a continuous variable.
To take into account the different recruitment periods, the multivariate models were also adjusted in accordance with a categorical variable corresponding to the recruitment periods (2002–2005, 2006–2009, 2010–2014).
GGT and ALP were analysed as continuous variables (logarithmic transformation) and as categorical variables: upper quartile and abnormally high values (GGT>36IU/L and ALP>120IU/L).
The following regression models were included: (1) Univariate. (2). Adjusted for age, sex, comorbidity score, diabetes, liver disease, active smoker, BMI, baseline eGFR and recruitment period. (3) Adjusted for the same variables as in model 2, plus total serum calcium, phosphorus, PTH, albumin, C-reactive protein, triglycerides, uric acid and alanine aminotransferase (ALT) levels. (4) Best fit model using the backward conditional stepwise elimination process.
The proportional hazards assumption was tested using log-minus-log curves and Schöenfeld residuals for each variable. Patients were censored at the time of death, loss to follow-up, renal transplant, or at the end of follow-up (31 December 2014), whichever occurred first.
Descriptive data are presented as a mean and standard deviation, or a median and interquartile range for continuous variables, and absolute values and percentages for categorical variables. A p-value <0.05 was considered statistically significant. Statistical analysis and graphs were produced using SPSS version 21.0 (IBM Corp., Armonk, NY, USA) and STATA 11.1 (StataCorp, TX, USA).
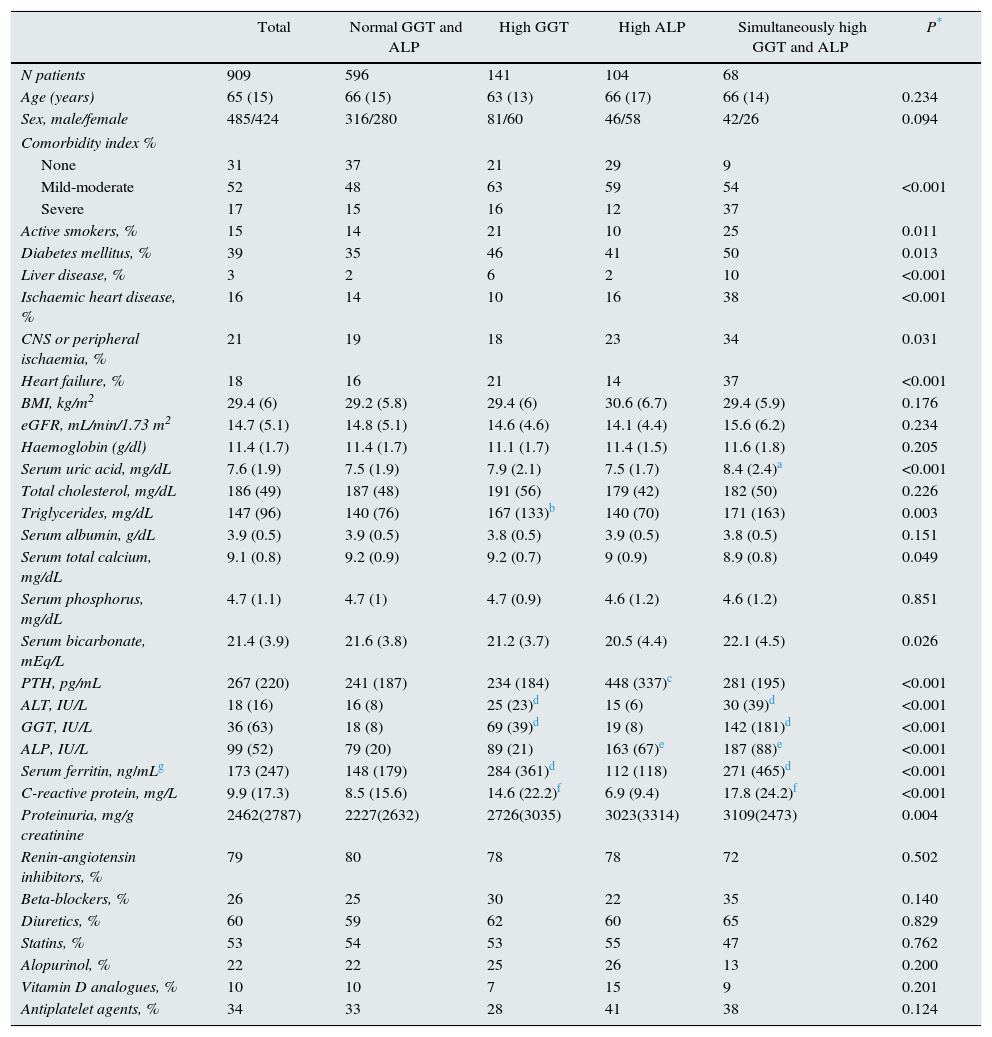
ResultsPatient characteristicsThe study group included 909 patients in CKD stage 4 or 5, pre-dialysis (mean eGFR 14.7±5.1mL/min/1.73m2). The mean age (±SD) was 65±15 years, and 53% of the patients were male. All were Caucasian. Baseline clinical and biochemical characteristics are shown in Table 1.
To analyse the patient clinical and biochemical characteristics according to the presence of abnormally high GGT or ALP levels, patients were divided into four sub-groups (Table 1): normal GGT and ALP levels (66% of patients), high GGT but normal ALP (16%), normal GGT but high ALP (11%), and simultaneously high GGT and ALP (7%).
Thus, 209 (23%) and 172 (19%) patients had abnormally high baseline GGT and ALP, respectively. The upper quartile value for GGT and ALP was 34IU/L and 112IU/L, respectively.
There were no significant differences in mean age and sex distribution among the sub-groups. The comorbidity score and the percent of active smokers was notably higher in patients with high GGT, especially in the group with simultaneously high GGT and ALP (Table 1).
Liver diseases were rare comorbidities in this set of patient. However, approximately 8% of patients with high GGT levels had been diagnosed with liver disease.
The prevalence of cardiovascular diseases in patients with either high GGT or high ALP was similar to that of patients with normal GGT and ALP values, however this prevalence of cardiovascular disease was significantly increased in patients with simultaneously high GGT and ALP.
Significant differences were observed in the biochemistry parameters among sub-groups (Table 1). Patients with high GGT had higher serum concentrations of uric acid, triglycerides, ALT, ferritin and CRP than patients with normal GGT and ALP levels, or high ALP alone. The sub-group of patients with high ALP alone had higher PTH levels. The mean baseline albumin values were similar among sub-groups.
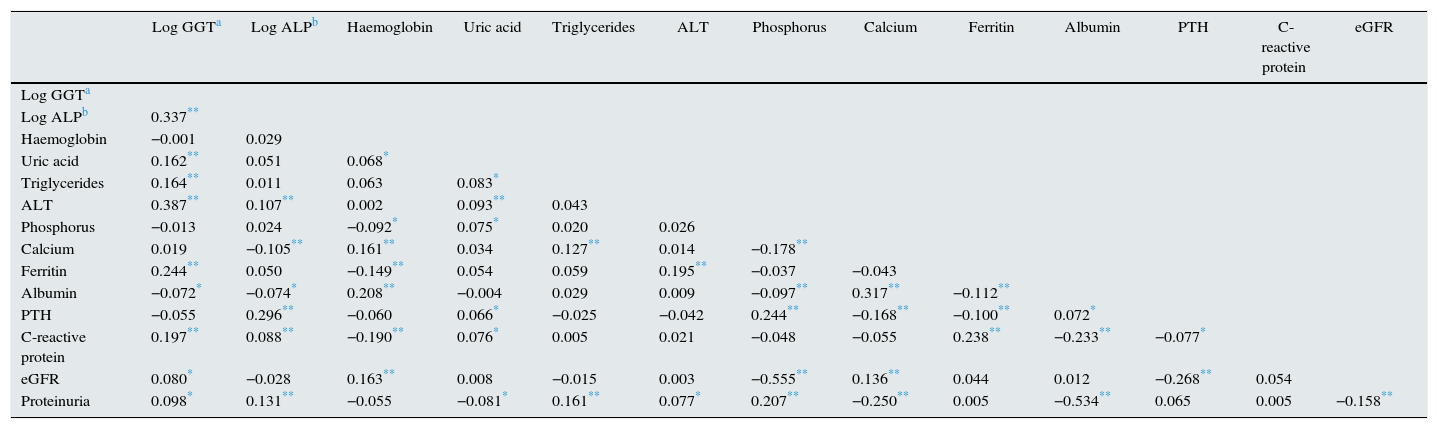
These biochemical patterns associated with GGT or ALP were confirmed by bivariate correlation analysis (Table 2).
Coefficients of correlation between biochemistry parameters.
| Log GGTa | Log ALPb | Haemoglobin | Uric acid | Triglycerides | ALT | Phosphorus | Calcium | Ferritin | Albumin | PTH | C-reactive protein | eGFR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log GGTa | |||||||||||||
| Log ALPb | 0.337** | ||||||||||||
| Haemoglobin | −0.001 | 0.029 | |||||||||||
| Uric acid | 0.162** | 0.051 | 0.068* | ||||||||||
| Triglycerides | 0.164** | 0.011 | 0.063 | 0.083* | |||||||||
| ALT | 0.387** | 0.107** | 0.002 | 0.093** | 0.043 | ||||||||
| Phosphorus | −0.013 | 0.024 | −0.092* | 0.075* | 0.020 | 0.026 | |||||||
| Calcium | 0.019 | −0.105** | 0.161** | 0.034 | 0.127** | 0.014 | −0.178** | ||||||
| Ferritin | 0.244** | 0.050 | −0.149** | 0.054 | 0.059 | 0.195** | −0.037 | −0.043 | |||||
| Albumin | −0.072* | −0.074* | 0.208** | −0.004 | 0.029 | 0.009 | −0.097** | 0.317** | −0.112** | ||||
| PTH | −0.055 | 0.296** | −0.060 | 0.066* | −0.025 | −0.042 | 0.244** | −0.168** | −0.100** | 0.072* | |||
| C-reactive protein | 0.197** | 0.088** | −0.190** | 0.076* | 0.005 | 0.021 | −0.048 | −0.055 | 0.238** | −0.233** | −0.077* | ||
| eGFR | 0.080* | −0.028 | 0.163** | 0.008 | −0.015 | 0.003 | −0.555** | 0.136** | 0.044 | 0.012 | −0.268** | 0.054 | |
| Proteinuria | 0.098* | 0.131** | −0.055 | −0.081* | 0.161** | 0.077* | 0.207** | −0.250** | 0.005 | −0.534** | 0.065 | 0.005 | −0.158** |
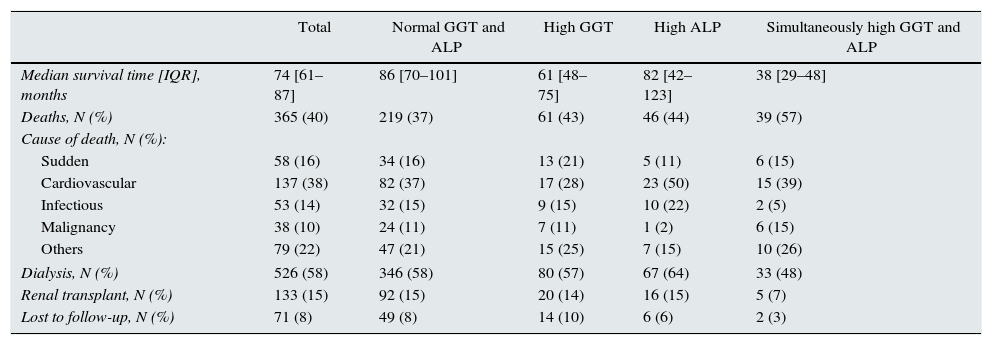
A total of 365 patients (40%) died during the study period, with a median survival for the patient set of 74 months (Table 3). As expected, the most common causes of death were cardiovascular and infection, with no significant differences among sub-groups (Table 3).
Mortality and other outcome variables of interest in the group overall and in subgroups according to GGT and alkaline phosphatase (ALP).
| Total | Normal GGT and ALP | High GGT | High ALP | Simultaneously high GGT and ALP | |
|---|---|---|---|---|---|
| Median survival time [IQR], months | 74 [61–87] | 86 [70–101] | 61 [48–75] | 82 [42–123] | 38 [29–48] |
| Deaths, N (%) | 365 (40) | 219 (37) | 61 (43) | 46 (44) | 39 (57) |
| Cause of death, N (%): | |||||
| Sudden | 58 (16) | 34 (16) | 13 (21) | 5 (11) | 6 (15) |
| Cardiovascular | 137 (38) | 82 (37) | 17 (28) | 23 (50) | 15 (39) |
| Infectious | 53 (14) | 32 (15) | 9 (15) | 10 (22) | 2 (5) |
| Malignancy | 38 (10) | 24 (11) | 7 (11) | 1 (2) | 6 (15) |
| Others | 79 (22) | 47 (21) | 15 (25) | 7 (15) | 10 (26) |
| Dialysis, N (%) | 526 (58) | 346 (58) | 80 (57) | 67 (64) | 33 (48) |
| Renal transplant, N (%) | 133 (15) | 92 (15) | 20 (14) | 16 (15) | 5 (7) |
| Lost to follow-up, N (%) | 71 (8) | 49 (8) | 14 (10) | 6 (6) | 2 (3) |
During the follow-up period, 58% of patients had to start dialysis, and 15% received a renal transplant (there were no pre-emptive transplants). No differences were observed either among sub-groups in the percentage of patients who started dialysis, underwent transplant, or were lost to follow-up (Table 3).
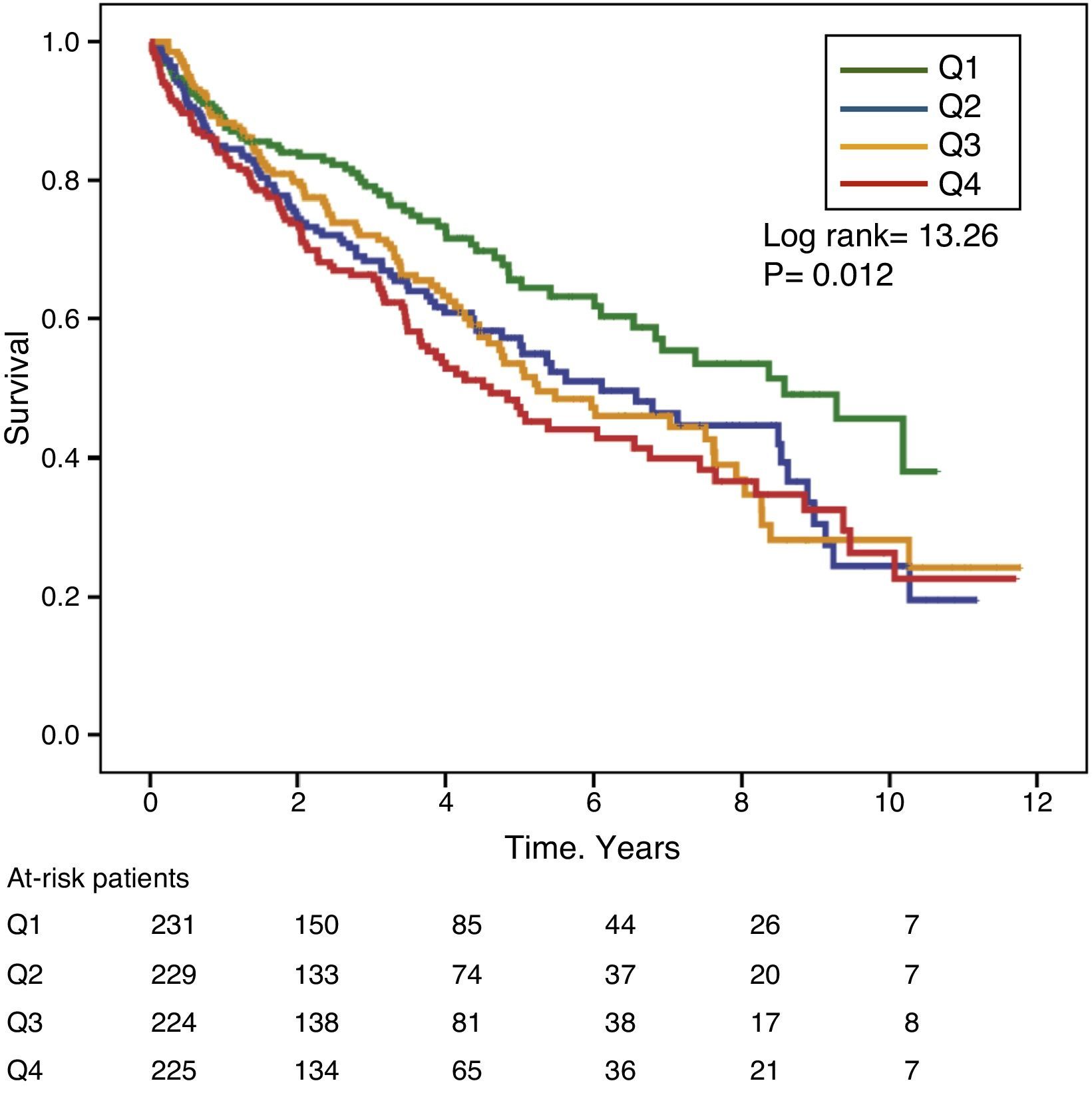
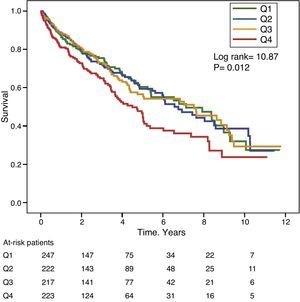
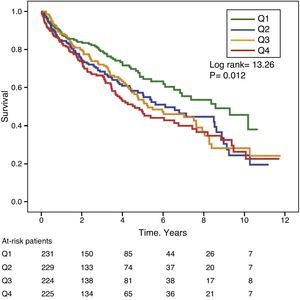
Gamma-glutamyl transferase and alkaline phosphatase as determinants of survivalFigs. 1 and 2 show the Kaplan–Meier survival curves according to the frequency distribution quartiles of the GGT and ALP values, respectively. Patients with high GGT or ALP levels (upper quartiles) had poorer survival.
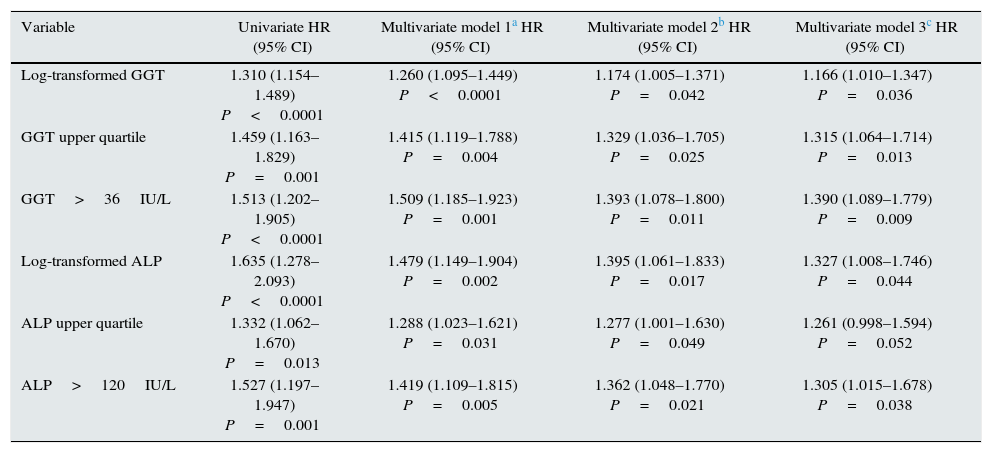
Using Cox regression models, GGT as a continuous or categorical variable (upper quartile or serum value >36IU/L) was significantly associated with increased mortality in the models analysed (Table 4). ALP was also significantly and independently associated with an increase in mortality in all models analysed, except if ALP was included in the best fit model as a categorical variable defined as the value of the upper quartile. In this model, the degree of association of ALP with mortality did not reach statistical significance (P=0.052) (Table 4).
Cox regression models of association with mortality.
| Variable | Univariate HR (95% CI) | Multivariate model 1a HR (95% CI) | Multivariate model 2b HR (95% CI) | Multivariate model 3c HR (95% CI) |
|---|---|---|---|---|
| Log-transformed GGT | 1.310 (1.154–1.489) P<0.0001 | 1.260 (1.095–1.449) P<0.0001 | 1.174 (1.005–1.371) P=0.042 | 1.166 (1.010–1.347) P=0.036 |
| GGT upper quartile | 1.459 (1.163–1.829) P=0.001 | 1.415 (1.119–1.788) P=0.004 | 1.329 (1.036–1.705) P=0.025 | 1.315 (1.064–1.714) P=0.013 |
| GGT>36IU/L | 1.513 (1.202–1.905) P<0.0001 | 1.509 (1.185–1.923) P=0.001 | 1.393 (1.078–1.800) P=0.011 | 1.390 (1.089–1.779) P=0.009 |
| Log-transformed ALP | 1.635 (1.278–2.093) P<0.0001 | 1.479 (1.149–1.904) P=0.002 | 1.395 (1.061–1.833) P=0.017 | 1.327 (1.008–1.746) P=0.044 |
| ALP upper quartile | 1.332 (1.062–1.670) P=0.013 | 1.288 (1.023–1.621) P=0.031 | 1.277 (1.001–1.630) P=0.049 | 1.261 (0.998–1.594) P=0.052 |
| ALP>120IU/L | 1.527 (1.197–1.947) P=0.001 | 1.419 (1.109–1.815) P=0.005 | 1.362 (1.048–1.770) P=0.021 | 1.305 (1.015–1.678) P=0.038 |
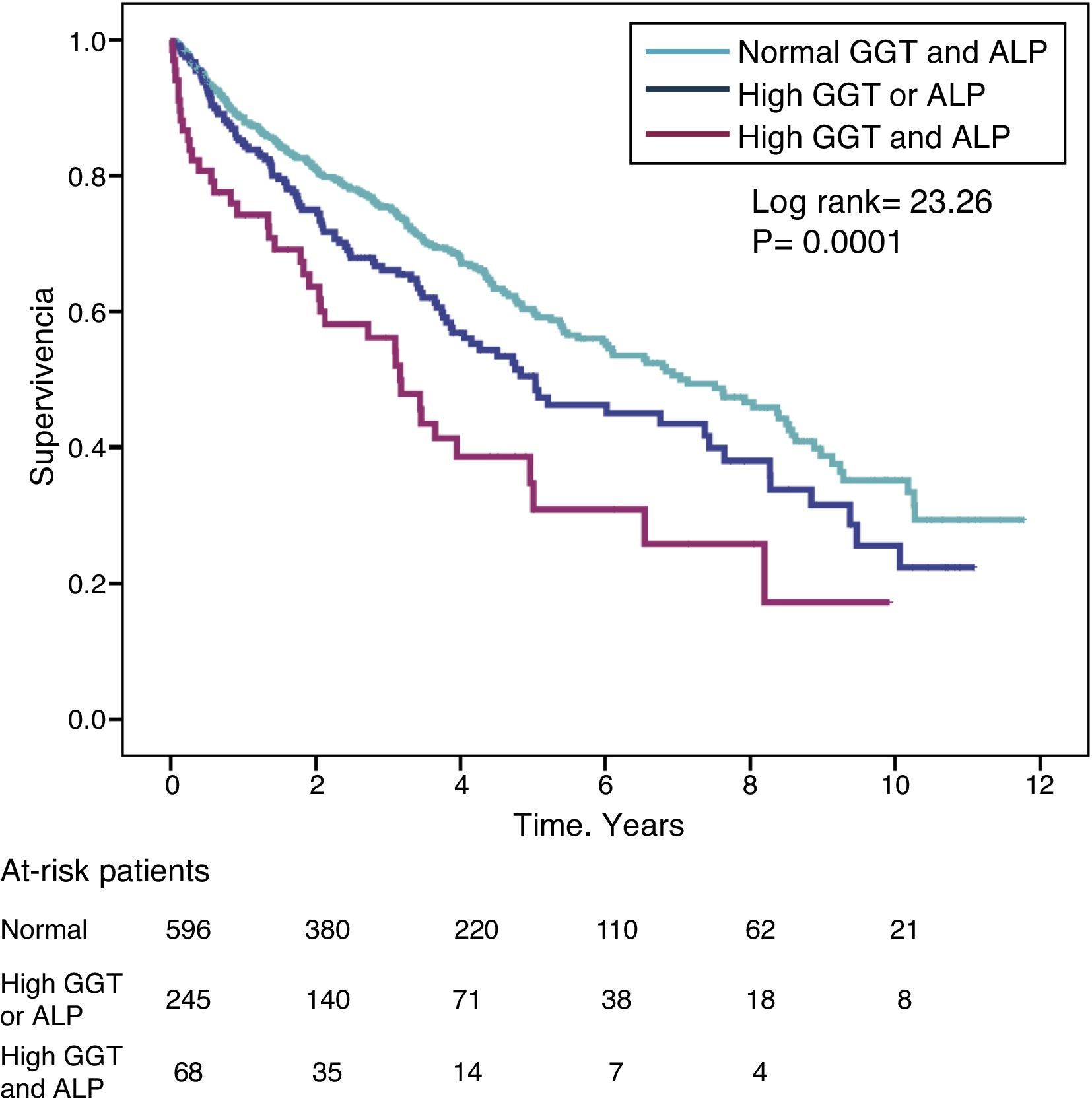
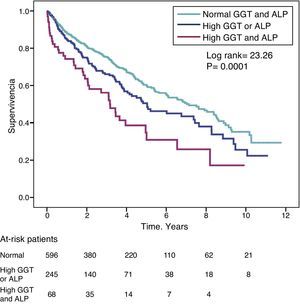
The hazard ratio (HR) for mortality in patients with high GGT levels (>36IU/L) (HR=1.39) was similar to that observed in patients with high ALP (>120IU/L) (HR=1.31) in the fully adjusted model (Table 4). Fig. 3 shows the Kaplan–Meier survival curves that illustrate this association, and the additive effect on reduced survival of simultaneously high GGT and ALP.
To evaluate the possible confounding effect of hepatic comorbidity on the association between GGT and ALP with mortality, a Cox regression model was constructed, excluding 27 patients previously diagnosed with liver disease. In this fitted model, high GGT (>36IU/L) maintained a statistically significant association with mortality (HR=1.316; 95% CI: 1.016–1.705; P=0.03), while high ALP (>120IU/L) showed an association at the limit of statistical significance (HR=1.296; 95% CI: 0.999–1.680; P=0.05).
DiscussionThe results of the present study show that elevated serum GGT or ALP is independently associated with higher mortality in patients with CKD. This study also reveals for the first time that the simultaneous elevation of both parameters has an additive effect on the prediction of mortality, even in patients without liver disease.
In this study, patients with CKD and high GGT levels had greater comorbidity, particularly diabetes and heart failure, and a biochemistry profile similar to that observed in metabolic syndrome, although the BMI in this subgroup was not different from that of the rest of the study patients.
Given the ubiquitous nature of ALP in the body, high serum levels of this enzyme can be an expression of different pathological processes. In this sense, determining specific ALP isoenzymes may be useful in distinguishing their origin in cases where isolated elevations cannot be attributed to increased bone remodelling.
The excess mortality reported in patients with CKD and high ALP levels has been controversially attributed to potential cardiovascular adverse effects as a result of very severe lack of control over bone-mineral metabolism.2,3,13,14 Several pathogenic mechanisms have been proposed to explain this association, including increased vascular calcification via pyrophosphate hydrolysis in the arterial wall,15 systemic inflammation16,17 and vitamin D deficiency.18 However, the absence of a significant association between serum levels of the bone-derived isoenzyme of ALP (bone-specific ALP) and mortality in patients with CKD19 raises serious doubts about the hypothesis on a pathogenic link with bone-mineral metabolism.
In this study, 11% of the patients had elevated ALP levels with normal GGT levels, and the main characteristic of this sub-group was the high mean PTH levels, suggesting that bone might be the origin of this ALP elevation. However, simultaneously high ALP and GGT characterised a sub-group of patients with a greater degree of comorbidity, mainly cardiovascular diseases, and a biochemistry profile that was more similar to that of patients with high GGT alone than to patients with high ALP alone. Thus, two risk profiles can be distinguished in patients with CKD and high ALP according to the presence or absence of high GGT levels. While high ALP alone was associated with a moderate risk of mortality, simultaneous elevation of both enzymes was associated with a higher risk of mortality.
GGT is the enzyme responsible for the hydrolysis of extracellular reduced glutathione (GSH), one of the main intracellular antioxidants in mammals, enabling the precursor amino acids to be subsequently used for new intracellular GSH synthesis.6 Thus, increased GGT is an expression of intracellular GSH depletion and, therefore, GGT could be considered as a marker of oxidative stress.6
Serum GGT has been shown to be a cardiometabolic biomarker in the general population. Various studies have found a significant, independent association between GGT levels and both cardiovascular and all-cause mortality.20–22 High GGT levels have been shown to be associated with a poorer prognosis in coronary artery disease and heart failure.23,24 A possible pathogenic implication of GGT in the formation of atherosclerotic plaque, its erosion and subsequent rupture has also been observed,25 which could help explain its relationship with cardiovascular morbidity and mortality from a biological point of view.
The predictive value of GGT in CKD patients has scarcely been studied. Postorino et al.9 observed a strong, independent association between high GGT levels and total and cardiovascular mortality in 584 CKD patients on dialysis. However, serum ALP concentrations were not determined in this study.
In another study, high GGT levels were associated with marked endothelial dysfunction in patients with CKD, which suggests a link between elevated levels of this marker and the risk of cardiovascular disease.10
In addition to the possible systemic pro-oxidative effect, other hypotheses could help to explain the association between elevated GGT levels and mortality in patients with CKD. Thus, high GGT could simply indicate alcohol abuse or liver diseases, comorbidities that would justify the excessive mortality. However, in this study, GGT maintained a significant association with mortality after excluding patients with chronic alcoholism, chronic liver diseases or hepatotropic virus infections, thus ruling out the exclusive role of liver disease in this association.
Nevertheless, fatty liver disease and congestive liver disease are two sub-clinical liver disorders that can cause high GGT levels.26,27 Fatty liver disease is a relatively common finding in patients with CKD, and is more closely related with cardiovascular risk factors than the development of liver complications.27 The clinical and biochemical characteristics associated with fatty liver disease are similar to those observed in the sub-group of patients in our study with high GGT. However, in this study, the diagnosis of fatty liver disease was not adequately studied in most patients and it therefore cannot be guaranteed that high GGT was mainly due to this disease.
Congestive liver disease refers to a spectrum of liver abnormalities attributed to the passive congestion secondary to right-sided heart failure or any cause that increases the central venous pressure, including volume overload, severe pulmonary hypertension or valvulopathies.28 Elevated serum GGT or ALP are characteristic biochemical abnormalities in CKD patients with left ventricular diastolic dysfunction, especially if they are associated with pulmonary congestion,29 which is also a recognised risk factor for mortality in these patients.30
Elevated GGT can also reflect microsomal enzyme induction.31 In addition to alcohol intake, many drugs can induce liver enzymes. In this study, none of the drugs most commonly prescribed in CKD patients were significantly associated with high GGT or ALP levels, although the potential effect of over-the-counter medicines (particularly analgesics) on high GGT levels in our patients cannot be ruled out.
This study has a series of limitations. Given its retrospective design, causal relationships could not be established. The study was conducted at a single site, and the cohort studied, although representative of the local population, was ethnically homogeneous (Caucasian). GGT and ALP were not analysed as time-varying covariates and, therefore, it is likely that the prognostic significance of transient increases in GGT is different to increases maintained over time. However, in this study, the detection of high GGT in the cross-sectional analysis at a certain stage of CKD was associated with a clinical phenotype of great interest due to its relationship with cardiovascular comorbidity and mortality. Finally, bone-specific ALP and other parameters related more specifically to bone remodelling were not measured.
In conclusion, an abnormally elevated GGT level is a common finding in patients with CKD, which is observed more commonly in patients with greater comorbidity, particularly cardiovascular diseases, and is independently associated with mortality. Simultaneously elevated serum GGT and ALP increase the mortality risk. Therefore, combined measurement of serum GGT with ALP could offer additional predictive information in patients with CKD.
Conflicts of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Caravaca-Fontán F, Azevedo L, Bayo MÁ, Gonzales-Candia B, Luna E, Caravaca F. Niveles séricos elevados de gamma-glutamil transferasa y fosfatasa alcalina son predictores independientes de mortalidad en la enfermedad renal crónica estadio 4-5. Nefrologia. 2017;37:267–275.