To estimate early mortality in patients with chronic kidney disease who started emergency haemodialysis between 2012 and 2014 in a national referral hospital in Lima, Peru, and to identify risk factors.
Design, characteristics, participants and measurementsA retrospective cohort study was conducted by reviewing the medical records of all patients admitted to the hospital's Haemodialysis Unit from 2012 to 2014. Early mortality, defined as death within the first 90 days of starting haemodialysis, as well as age, gender, chronic kidney disease aetiology, comorbidities, cause of death, estimated glomerular filtration rate, vascular access and other variables were evaluated in patients who initiated emergency haemodialysis. Early mortality was estimated using frequencies, and risk factors were determined by Poisson regression with robust variance.
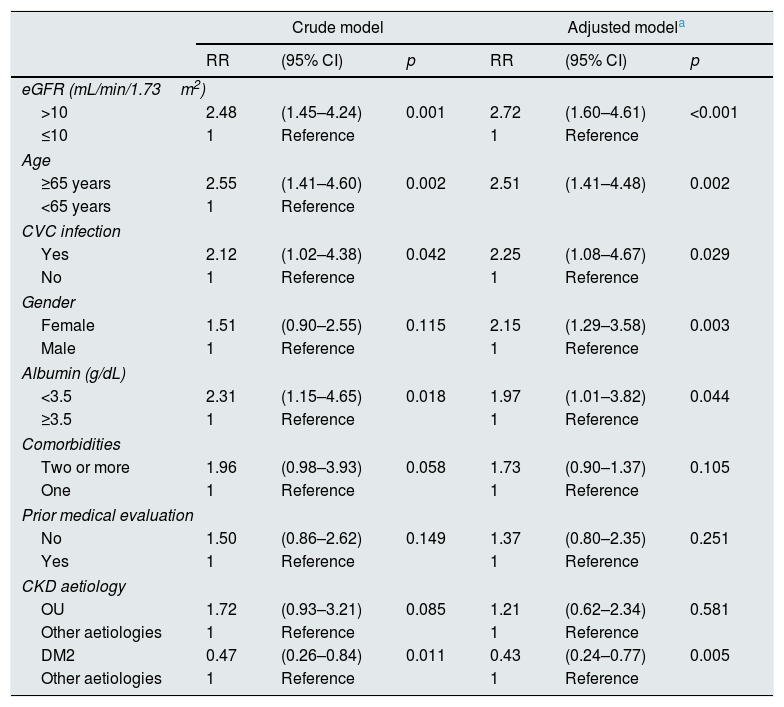
Results43.4% of patients were female, 51.5% were aged ≥65 years and the early mortality rate was 9.3%. The main risk factors were estimated glomerular filtration rate >10mL/min/1.73m2 (RR: 2.72 [95% CI: 1.60–4.61]); age ≥65 years (RR: 2.51 [95% CI: 1.41–4.48]); central venous catheter infection, RR: 2.25 (95% CI: 1.08–4.67); female gender, RR: 2.15 (95% CI: 1.29–3.58); and albumin<3.5g/dL (RR: 1.97 [95% CI: 1.01–3.82]).
ConclusionsEarly mortality was 9.3%. The main risk factor was starting haemodialysis with an estimated glomerular filtration rate >10mL/min/1.73m2.
Estimar la mortalidad precoz en pacientes con enfermedad renal crónica que iniciaron hemodiálisis por urgencia entre los años 2012-2014 en un hospital de referencia nacional en Lima, Perú, e identificar los factores de riesgo.
Diseño, características, participantes y medicionesSe estudió una cohorte retrospectiva mediante la revisión de historias clínicas de todos los pacientes admitidos a la Unidad de Hemodiálisis del hospital en el periodo de tiempo señalado. Se evaluó mortalidad precoz, definida como la muerte dentro de los primeros 90 días luego de iniciar hemodiálisis, así como edad, sexo, etiología de enfermedad renal crónica, comorbilidades, causa de muerte, tasa de filtración glomerular estimada, acceso vascular, entre otras variables, en los pacientes que iniciaron hemodiálisis por urgencia. Se estimó la mortalidad precoz mediante frecuencias y se utilizó regresión de Poisson con varianza robusta para determinar los factores de riesgo.
ResultadosSe encontró que el 43,4% fueron mujeres, el 51,5% tenían≥65 años y una mortalidad precoz del 9,3%. Los principales factores de riesgo fueron tasa de filtración glomerular estimada>10mL/min/1,73m2 (RR: 2,72 [IC 95%: 1,60-4,61]); edad≥65 años (RR: 2,51 [IC 95%: 1,41-4,48]); infección de catéter venoso central, RR: 2,25 (IC 95%: 1,08–4,67); sexo femenino, RR: 2,15 (IC 95%: 1,29–3,58); y albúmina<3,5g/dL (RR: 1,97 [IC 95%: 1,01–3,82]).
ConclusionesLa mortalidad precoz fue del 9,3%. El principal factor de riesgo fue iniciar hemodiálisis con una tasa de filtración glomerular estimada>10mL/min/1,73m2.
Chronic kidney disease (CKD) is a major health problem worldwide. In some countries, it affects up to 8.1% of the total population.1 The number of new cases, and the use of renal replacement therapies (RRTs), has increased in recent years, but not in equal proportion.2,3 It is estimated that, by 2030, the number of patients starting some form of RRT globally will be more than double with respect to 2010, reaching up to 5.5 million people.2 In Latin America, the prevalence of patients with stage 5 CKD undergoing some form of RRT increased from 119 patients per million population (pmp) in 1991 to 660 patients pmp in 2010. Here, haemodialysis continues to be the most commonly used form of RRT compared to the other therapies (75% of patients).4 It has been calculated that, in Peru, between 20,000 and 40,000 patients need some form of RRT.5 However, according to the Analysis of the CKD situation in Peru for 2015, 415 patients pmp received some form of RRT. The Peruvian Social Security System (EsSalud) provides some form of RRT to 78.5% of these patients, unlike the Ministry of Health (MINSA), which only covers 5.3%.6 The most recent report from the United States Renal Data System (USRDS) for 2014 revealed an annual mortality rate in patients with CKD of approximately 14%.7 The majority of studies evaluating the annual mortality rate of patients with CKD undergoing haemodialysis do not include the first 90 days after starting therapy, since it is considered to be associated with haemodialysis itself and with the natural progression of the illness that caused it.8 Since 2013, the USRDS has included the first 90 days in its mortality studies in order to also evaluate the incidence in this time interval.9,10 Many studies have defined death occurring within this period of time as “early mortality”.11–14 In the last report from 2014, a significant peak in mortality was observed (8.6%) between the second and third months after starting haemodialysis.7 Others have reported an early mortality rate ranging between 4 and 12%, similar to the latest report from the USRDS.11–14 Among the risk factors for early mortality, Ortega et al.15 identified the starting of emergency dialysis, temporary catheter infection and serum albumin <3.5g/dL. McQuillan et al. found that the patient's nutritional status, the pre-dialysis nephrology care and the type of vascular access used at the start of haemodialysis represented modifiable factors that could prevent early mortality.14 Peruvian studies did not find significant differences on evaluating factors associated with early mortality.16 Some of the most common causes included those of cardiovascular origin (34.2%), such as acute myocardial infarction, and cerebrovascular accident (stroke) and sepsis (13.8%).14 In view of the above, and considering that previous studies in the country have included a small number of participants, the early mortality of patients with CKD who started emergency haemodialysis is currently a topic of interest in the management of pre-dialysis patients.
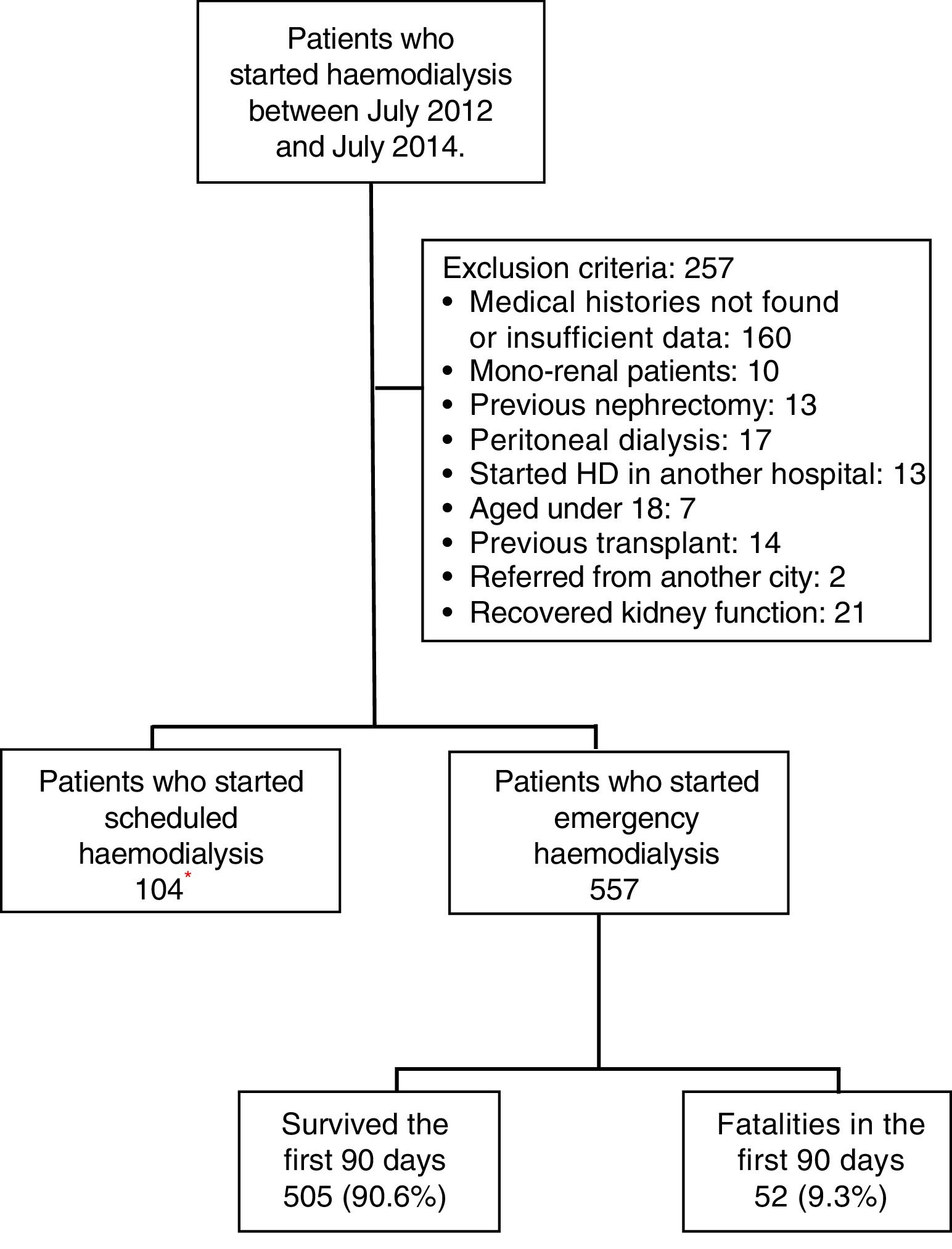
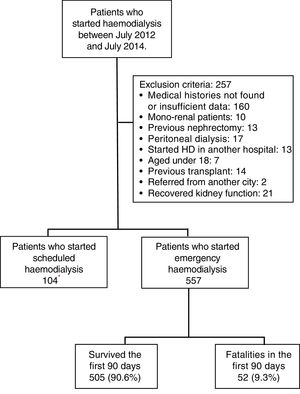
MethodologyA retrospective cohort was studied by evaluating the medical histories of patients who were admitted on an emergency basis to the Haemodialysis Unit of the Hospital Nacional Edgardo Rebagliati Martins (HNERM) between July 2012 and July 2014. The HNERM is an EsSalud reference hospital in Lima, Peru which receives patients with CKD who need to start haemodialysis from the entire Rebagliati Healthcare Network, which has a total allocated population of 1,871,566 patients.17 To do this, a census was conducted on 918 patients who started haemodialysis during the time period mentioned. The indication upon admission for emergency haemodialysis was decided by the attending physician based on clinical and laboratory criteria. The criteria included uraemic encephalopathy, acute pulmonary oedema, uraemic gastropathy, oligoanuria, uraemic pericarditis, hyperkalaemia, and medically treated refractory metabolic acidosis. Those patients who had medical histories with insufficient data or which were not found after up to 2 searches were excluded. Furthermore, other patients excluded were those who started haemodialysis in another hospital, under 18 years of age, those referred from other cities, mono-renal patients, with a history of peritoneal dialysis, nephrectomy or kidney transplant, and those who recovered kidney function, as recorded in the medical history (Fig. 1). At the end, 557 patients were enrolled in the study. All variables were obtained from the medical records of the patients evaluated or from the Haemodialysis Unit database. The date of death was obtained from the HNERM death records. In order to evaluate early mortality, defined as the death of the patient within the first 90 days after the start of haemodialysis,11–15 the list of patients who started haemodialysis was compared with the death records on the HNERM IT system for the corresponding years. The age, sex, haemodialysis start date, CKD aetiology, presence of comorbidities and the type of vascular access used were also recorded according to medical history records or the HNERM Haemodialysis Unit database. The comorbidities included the presence of acute myocardial infarction, heart failure, peripheral vascular disease, stroke, dyslipidaemia, neoplasia, chronic obstructive pulmonary disease, arrhythmia, and obstructive uropathy, among others. With respect to the central venous catheter (CVC), a temporary CVC was used. A patient with a CVC infection was defined according to that recorded in the medical history. Clinical infection data and the presence of a positive culture of the same bacteria in samples obtained from the catheter tip and blood culture was considered; however, the microorganism causing the infection was not included in the study. The cause of death was determined by the attending physician according to clinical and laboratory criteria, and obtained from the epicrisis present in the medical history. The absence or presence of a prior medical evaluation, whether by a nephrologist alone or a multidisciplinary medical team (nephrologist, cardiologist, endocrinologist, cardiovascular surgeon and nutritionist), and the time at which it was conducted (more or less than 30 days before starting haemodialysis) were evaluated. Furthermore, the results of laboratory examinations conducted within the 24h prior to starting haemodialysis were included: haemoglobin (g/dL), glucose (mg/dL), urea (mg/dL), creatinine (mg/dL), albumin (g/dL), calcium (g/dL), phosphorous (g/dL), cholesterol (mg/dL), triglycerides (mg/dL) and parathormone (pg/mL), according to that recorded in the medical history. The estimated glomerular filtration rate (eGFR) was calculated using the MDRD 4® formula and was categorised as ≤10 and >10mL/min/1.73m2.18–21 All the variables were obtained from the medical histories of the patients evaluated or from the Haemodialysis Unit database. The date of death was obtained from the HNERM death records. The Microsoft Excel 2010® program was used to prepare the database and, in order to ensure the quality of the database, double data entry was used. The data analysis was conducted using the STATA 14® program. To describe the characteristics of the population, absolute and relative frequencies were used in the categorical variables and median values and the interquartile range were used in the numeric variables. Similarly, early mortality and the main causes of death were calculated using frequencies. The Chi-squared and Fisher's exact statistical tests were used for the bivariate analysis. Those values that had a p-value <0.20 were entered into the multivariate model. This was done using Poisson regression with a robust variance. This study was approved by the Ethics Committee of the Universidad Peruana de Ciencias Aplicadas [Peruvian University of Applied Sciences] and the HNERM. Furthermore, only medical history codes were used for the collection of data in order to maintain patient anonymity.
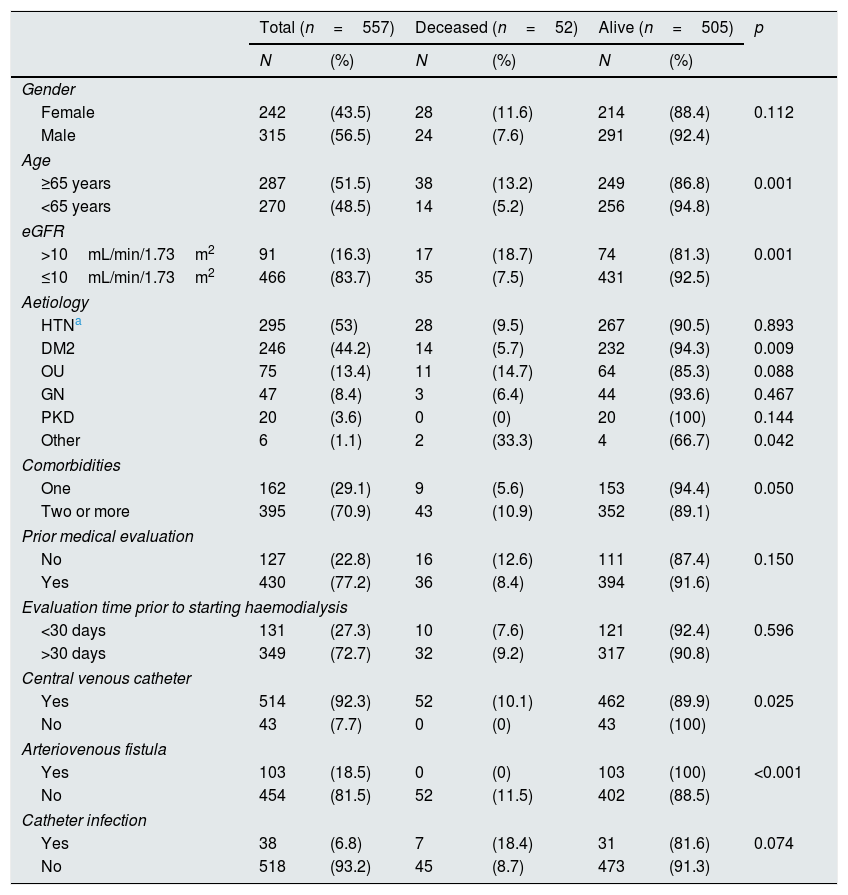
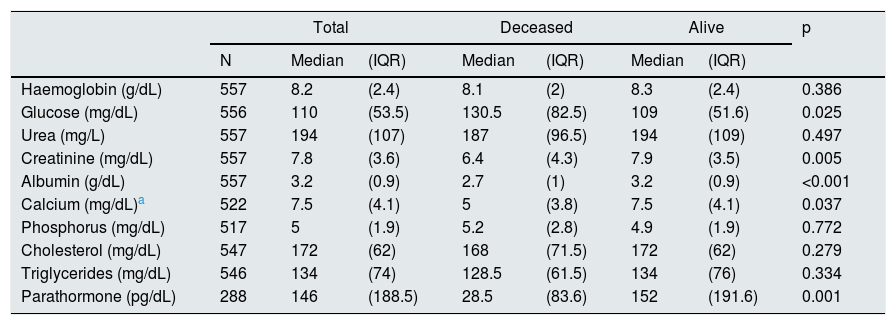
ResultsBetween July 2012 and July 2014, 918 patients were admitted to the HNERM Haemodialysis Unit. Around 250 patients met the exclusion criteria and did not participate in the study. Of those remaining, 557 patients started emergency haemodialysis, as seen in Fig. 1. A, 43.5% were women and 51.5% were ≥65-years-old. The most common aetiology of CKD was hypertension. More than two-thirds of patients presented 2 or more comorbidities and the eGFR in 16.3% was ≥10mL/min/1.73m2. Regarding the pre-dialysis management, 22.8% were never evaluated by a nephrologist or a multidisciplinary medical team, while, of the patients who underwent a medical evaluation, 72.7% were evaluated more than 30 days before the start of haemodialysis. The most commonly used vascular access was CVC (92.3%) and 6.8% presented an infection of the CVC. Comparison of the previously mentioned variables in patients who survived vs those who died are presented in Table 1. Of the 557 patients undergoing haemodialysis, 9.3% died within the first 90 days after starting haemodialysis. The main causes of early mortality were infections (59.6%), the majority of which were respiratory or urinary, followed by acute myocardial infarction (15.4%), neoplasia (11.5%), stroke (9.6%) and arrhythmias (1.9%). In terms of the laboratory variables, the group of patients who died within the first 90 days of beginning haemodialysis had a lower median of haemoglobin, albumin, urea, creatinine, calcium, parathormone, cholesterol and triglycerides as compared to those who survived, this is not true for glucose and phosphorus values (Table 2). In the adjusted multivariate analysis, it was revealed that those patients with an eGFR >10mL/min/1.73m2 (RR: 2.72 [95% CI: 1.60–4.61]); ≥ 65 years (RR: 2.51 [95% CI: 1.41–4.48]); with CVC infection, RR: 2.25 (95% CI: 1.08–4.67); who were females, RR: 2.15 (95% CI: 1.29–3.58) and had albumin <3.5g/dL (RR: 1.97 [95% CI: 1.01–3.82]) had a greater risk of early mortality (Table 3).
Characteristics of patients who started emergency haemodialysis between July 2012 and July 2014 in the HNERM.
| Total (n=557) | Deceased (n=52) | Alive (n=505) | p | ||||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | ||
| Gender | |||||||
| Female | 242 | (43.5) | 28 | (11.6) | 214 | (88.4) | 0.112 |
| Male | 315 | (56.5) | 24 | (7.6) | 291 | (92.4) | |
| Age | |||||||
| ≥65 years | 287 | (51.5) | 38 | (13.2) | 249 | (86.8) | 0.001 |
| <65 years | 270 | (48.5) | 14 | (5.2) | 256 | (94.8) | |
| eGFR | |||||||
| >10mL/min/1.73m2 | 91 | (16.3) | 17 | (18.7) | 74 | (81.3) | 0.001 |
| ≤10mL/min/1.73m2 | 466 | (83.7) | 35 | (7.5) | 431 | (92.5) | |
| Aetiology | |||||||
| HTNa | 295 | (53) | 28 | (9.5) | 267 | (90.5) | 0.893 |
| DM2 | 246 | (44.2) | 14 | (5.7) | 232 | (94.3) | 0.009 |
| OU | 75 | (13.4) | 11 | (14.7) | 64 | (85.3) | 0.088 |
| GN | 47 | (8.4) | 3 | (6.4) | 44 | (93.6) | 0.467 |
| PKD | 20 | (3.6) | 0 | (0) | 20 | (100) | 0.144 |
| Other | 6 | (1.1) | 2 | (33.3) | 4 | (66.7) | 0.042 |
| Comorbidities | |||||||
| One | 162 | (29.1) | 9 | (5.6) | 153 | (94.4) | 0.050 |
| Two or more | 395 | (70.9) | 43 | (10.9) | 352 | (89.1) | |
| Prior medical evaluation | |||||||
| No | 127 | (22.8) | 16 | (12.6) | 111 | (87.4) | 0.150 |
| Yes | 430 | (77.2) | 36 | (8.4) | 394 | (91.6) | |
| Evaluation time prior to starting haemodialysis | |||||||
| <30 days | 131 | (27.3) | 10 | (7.6) | 121 | (92.4) | 0.596 |
| >30 days | 349 | (72.7) | 32 | (9.2) | 317 | (90.8) | |
| Central venous catheter | |||||||
| Yes | 514 | (92.3) | 52 | (10.1) | 462 | (89.9) | 0.025 |
| No | 43 | (7.7) | 0 | (0) | 43 | (100) | |
| Arteriovenous fistula | |||||||
| Yes | 103 | (18.5) | 0 | (0) | 103 | (100) | <0.001 |
| No | 454 | (81.5) | 52 | (11.5) | 402 | (88.5) | |
| Catheter infection | |||||||
| Yes | 38 | (6.8) | 7 | (18.4) | 31 | (81.6) | 0.074 |
| No | 518 | (93.2) | 45 | (8.7) | 473 | (91.3) | |
DM2: diabetes mellitus type 2; eGFR: estimated glomerular filtration rate; GN: glomerulonephritis; HNERM: Hospital Nacional Edgardo Rebagliati Martins; HTN: hypertension; OU: obstructive uropathy; PKD: polycystic kidney disease.
Characteristics of the laboratory values of patients who started emergency haemodialysis between July 2012 and July 2014 in the HNERM.
| Total | Deceased | Alive | p | |||||
|---|---|---|---|---|---|---|---|---|
| N | Median | (IQR) | Median | (IQR) | Median | (IQR) | ||
| Haemoglobin (g/dL) | 557 | 8.2 | (2.4) | 8.1 | (2) | 8.3 | (2.4) | 0.386 |
| Glucose (mg/dL) | 556 | 110 | (53.5) | 130.5 | (82.5) | 109 | (51.6) | 0.025 |
| Urea (mg/L) | 557 | 194 | (107) | 187 | (96.5) | 194 | (109) | 0.497 |
| Creatinine (mg/dL) | 557 | 7.8 | (3.6) | 6.4 | (4.3) | 7.9 | (3.5) | 0.005 |
| Albumin (g/dL) | 557 | 3.2 | (0.9) | 2.7 | (1) | 3.2 | (0.9) | <0.001 |
| Calcium (mg/dL)a | 522 | 7.5 | (4.1) | 5 | (3.8) | 7.5 | (4.1) | 0.037 |
| Phosphorus (mg/dL) | 517 | 5 | (1.9) | 5.2 | (2.8) | 4.9 | (1.9) | 0.772 |
| Cholesterol (mg/dL) | 547 | 172 | (62) | 168 | (71.5) | 172 | (62) | 0.279 |
| Triglycerides (mg/dL) | 546 | 134 | (74) | 128.5 | (61.5) | 134 | (76) | 0.334 |
| Parathormone (pg/dL) | 288 | 146 | (188.5) | 28.5 | (83.6) | 152 | (191.6) | 0.001 |
HNERM: Hospital Nacional Edgardo Rebagliati Martins; IQR: interquartile range.
Early mortality risk factors in patients who started emergency haemodialysis between July 2012 and July 2014 in the HNERM.
| Crude model | Adjusted modela | |||||
|---|---|---|---|---|---|---|
| RR | (95% CI) | p | RR | (95% CI) | p | |
| eGFR (mL/min/1.73m2) | ||||||
| >10 | 2.48 | (1.45–4.24) | 0.001 | 2.72 | (1.60–4.61) | <0.001 |
| ≤10 | 1 | Reference | 1 | Reference | ||
| Age | ||||||
| ≥65 years | 2.55 | (1.41–4.60) | 0.002 | 2.51 | (1.41–4.48) | 0.002 |
| <65 years | 1 | Reference | ||||
| CVC infection | ||||||
| Yes | 2.12 | (1.02–4.38) | 0.042 | 2.25 | (1.08–4.67) | 0.029 |
| No | 1 | Reference | 1 | Reference | ||
| Gender | ||||||
| Female | 1.51 | (0.90–2.55) | 0.115 | 2.15 | (1.29–3.58) | 0.003 |
| Male | 1 | Reference | 1 | Reference | ||
| Albumin (g/dL) | ||||||
| <3.5 | 2.31 | (1.15–4.65) | 0.018 | 1.97 | (1.01–3.82) | 0.044 |
| ≥3.5 | 1 | Reference | 1 | Reference | ||
| Comorbidities | ||||||
| Two or more | 1.96 | (0.98–3.93) | 0.058 | 1.73 | (0.90–1.37) | 0.105 |
| One | 1 | Reference | 1 | Reference | ||
| Prior medical evaluation | ||||||
| No | 1.50 | (0.86–2.62) | 0.149 | 1.37 | (0.80–2.35) | 0.251 |
| Yes | 1 | Reference | 1 | Reference | ||
| CKD aetiology | ||||||
| OU | 1.72 | (0.93–3.21) | 0.085 | 1.21 | (0.62–2.34) | 0.581 |
| Other aetiologies | 1 | Reference | 1 | Reference | ||
| DM2 | 0.47 | (0.26–0.84) | 0.011 | 0.43 | (0.24–0.77) | 0.005 |
| Other aetiologies | 1 | Reference | 1 | Reference | ||
95% CI: 95% confidence interval; CKD: chronic kidney disease; CVC: central venous catheter; DM2: diabetes mellitus type 2; eGFR: estimated glomerular filtration rate; HNERM: Hospital Nacional Edgardo Rebagliati Martins; OU: obstructive uropathy; RR: relative risk.
The main outcome was that an early mortality rate of 9.3% was found in patients who started emergency haemodialysis in the HNREM between July 2012 and July 2014. In recent years, Noordzij and Jager22 compared the haemodialysis admission records of the United States (USRDS), Canada (Canadian Organ Replacement Register) and Europe (European Renal Association-European Dialysis and Transplant Association) and found early mortality rates of 8.6%, 5.6% and 6.6%, respectively, which represent around 40% of that occurring in the first year after starting haemodialysis. Similar studies conducted around 1990 showed similar values.11,13,14,23–25 In Japan, Yamagata et al.26 reported a mortality rate of 17%, higher when compared to the countries mentioned27 and similar to the data for Colombia (17.5%).15 The present study found an early mortality rate similar to that of the developed countries; however, these were different from other studies reported in Peru. In 2015, Herrera-Añazco et al. found a 90-day mortality rate of 37.7% in patients who started haemodialysis in the Hospital Nacional Dos de Mayo, a MINSA national reference hospital, which represents 4 times more than the result of this study.28 Two years before, Herrera-Añazco et al. reported a mortality rate of 23.3% during the first hospitalisation period in the same population.29 These differences could be explained by the inequalities existing between both healthcare systems. EsSalud covers approximately 20% of the Peruvian population, unlike MINSA, which covers around 70%.30 However, EsSalud treats the majority of patients in the country who require some form of RRT, since it has haemodialysis units with a greater resolution capacity, with 135 operational machines pmp, distributed throughout the majority of cities, in contrast with MINSA which offers 15 operational machines pmp, and which are mainly found in the capital city.6 Furthermore, EsSalud has CKD patient management protocols, whereas MINSA does not.28 A significant share of controlling the progression of CKD lies in how aware the patient is about his/her disease and the measures that he/she can take to slow down CKD progression. A study published by Solís et al. found that the population allocated to MINSA presented a lower educational level and had poorer living conditions compared to EsSalud and armed forces patients, which meant that this population was more likely to seek medical care at a later stage.31 Furthermore, this group of individuals who are not insured under EsSalud, whether due to not having stable employment, being underemployed, or belonging to certain vulnerable populations, and, despite presenting advanced stages of CKD, have to wait until they are in critical condition to go to a MINSA healthcare institution. Even then, there is no guarantee that they will find a space available for starting emergency dialysis. This leads us to infer that the number of patients on dialysis in MINSA is not representative of the assigned population, and the critical condition in which they arrive shows an unrealistically high early mortality rate. Regarding the risk factors for early mortality, starting haemodialysis with an eGFR >10mL/min/1.73m2 represented a 2.72-fold higher risk of dying in the first 90 days. In the last decade, several studies have been published that show similar results. In 2012, a meta-analysis that included 16 cohort studies and a controlled study revealed that higher eGFRs (for every 1mL/min/1.73m2) were associated with a higher all-cause mortality.32 This could be due to the fact that those patients with higher residual renal function on starting haemodialysis, i.e., those who preserved part of the clearance mechanism, cleared a certain amount of the exchange dialysis volume. Therefore, they present a higher risk of developing complications attributed to the ultrafiltration treatment itself, such as the onset of hypotension.33 Furthermore, Noordzij and Jager22 and Rognant and Laville34 suggested that some of the patients who started haemodialysis with higher eGFRs did so because they presented a rapidly progressing compromised health status and who, despite haemodialysis, did not survive. It has also been observed that starting this RRT becomes a limiting factor in terms of the quality of life of patients,35 and that it even increases the intention of abandoning treatment up to two-fold.36 Given that there is still no clear explanation of this link, studies are required to substantiate the hypotheses for why starting haemodialysis with higher eGFR values are associated with higher mortality, as well as the potential implications of starting haemodialysis later. Other risk factors were found in addition to the eGFR. Patients who presented a CVC infection had a 2.25-fold greater risk of dying within the first 90 days of starting haemodialysis. Ortega et al. found, in their bivariate analysis, that a catheter-related infection was associated with a 2.44-fold higher risk of dying.15 It is important that future studies identify the type of bacteria causing the CVC infection due to the differences existing between community-acquired and hospital-acquired infections, and, as regards the latter, even according to whether they are acquired in hospital environments or critical care departments. It should be noted that, within the adjusted model of the statistical analysis, the type of vascular access used could not be included, since, during the follow-up, none of the patients with an arteriovenous fistula died, and at least one case must have an event of interest so that there is no error in the modelling. In the HNERM, the indication for creating a permanent vascular access arises as part of the execution of the CKD patient management protocol, which indicates that the patients with an arteriovenous fistula had already been evaluated by a nephrologist during the evolution of their disease. It could be inferred that, rather than the type of vascular access present at the time haemodialysis begins, regardless of whether it is an arteriovenous fistula or CVC, it is the access to a nephrological medical evaluation that could decrease the risk of early mortality in these patients, as other studies have confirmed.37,38 Women and people ≥65-years-old also had more than double the risk of dying within the first 3 months after starting haemodialysis. In terms of gender, the results reported varied. Some studies reported that the female population had a greater risk of dying,39 while others reported that it was men who had a greater risk.13,14,40 With respect to age, various studies, both national and international, assert that the older you are the more at risk you are of early mortality.11,12,15,23,25,28,41 This could be explained by the unpredictable course of the progression of CKD presented by these patients, the signs of uraemia that are independent of their eGFR value, and the higher independent risk of death that they present as compared to young patients.42,43 Regarding albumin values, the presence of hypoalbuminaemia constitutes an almost 2-fold higher risk of mortality; this is supported by other studies that show that values ≥3.5g/dL have higher rates of survival, not only during the first 90 days from the start of haemodialysis, but also in the long term.44 The measure of albumin is a way of measuring the nutritional status. It is known that an older adult population presents with protein-energy wasting syndrome45 more commonly, and, accordingly, they start haemodialysis with albumin values <3.5g/dL, similar to that observed in this study. As regards diabetes mellitus type 2 as a CKD aetiology, it was revealed that patients who presented with this disease had a lower risk of death, unlike that reported in numerous studies.46,47 This controversial result needs to be verified by other studies. Among the possible explanations, we believe that they could be related more to the management of the disease than to its effects, since diabetic patients had more medical tests with nephrologists and started dialysis with lower eGFR values, which could impact on these findings.38 Furthermore, the specific causes of death included infections and cardiovascular problems (acute myocardial infarction, stroke and arrhythmias), followed by cancer. These findings correspond with the recent study by Wick et al. which created a clinical prediction rule for early death in haemodialysis, where having metastatic cancer is one of the criteria with the highest risk of mortality, as well as being older and having a higher eGFR. These results are consistent with this study.48 In terms of the limitations, this study was conducted in only one hospital, which belongs to EsSalud, and it does not consider those patients treated by MINSA, the armed forces, the police or private clinics. As such, the results obtained cannot be extrapolated. However, the HNREM is a national reference hospital, has the greatest allocated amount of patients insured through EsSalud and takes care of the majority of haemodialysis patients in the country.15 Therefore, the results obtained can be considered a baseline study for future multicentre investigations. With respect to the estimated GFR, the MDRD 4® formula was used. Although this is not the best tool to evaluate kidney function, since the creatinine values are influenced by extrinsic variables, such as the sarcopenia observed in older patients,32 it is the most commonly used tool in emergency departments due to its practicality. It is important to mention that the population studied was considered “non-black”, since race information did not appear in the medical history. It should be stated that the same modelling was done using the CKD–EPI creatinine equation and the results were the same in terms of the variables that were found to be significant in the final model. Another significant limitation was that, as this was a retrospective study that used secondary information sources, there were a lot of patients who were excluded due to not having all the variables of interest, which is why variables, such as calcium, phosphorus, and parathormone, were not measured in the multivariate analysis. However, no differences were found between the included and excluded populations when the characteristics of both groups were compared. Future prospective studies should be conducted to corroborate the findings and should also include variables such as the reasons for emergency dialysis, in order to determine which have the highest probability of death, so that these can be more closely monitored. The results obtained in the HNERM, similar to developed countries, help to determine that the clinical management protocol used is a first step in increasing survival in this group of patients. However, there are aspects of the protocol that can still be improved. In recent years, there has been a tendency to note that starting haemodialysis with a higher eGFR represents a higher risk of early mortality; however, an exact eGFR from which some form of RRT should be started has still not been identified. It is recommended that each patient is comprehensively evaluated, decisions are made on an individual basis and that the risk profile of patients with CKD, in terms of death in the first 90 days after starting emergency haemodialysis, is considered.
ConclusionsIn conclusion, 9.3% of patients who started emergency haemodialysis between July 2012 and July 2014 in the HNERM died during the first 90 days. Those who started haemodialysis with an eGFR >10mL/min/1.73m2, who were older than 65-years-old, female, had a CVC infection and hypoalbuminaemia (albumin <3.5g/dL), have a higher risk of early mortality.
Conflict of interestThe authors declare that they have no conflicts of interests.
Please cite this article as: Gómez de la Torre-del Carpio A, Bocanegra-Jesúsa A, Guinetti-Ortiza K, Mayta-Tristánb P, Valdivia-Vegaa R. Impacto pronóstico a largo plazo de la anticoagulación en pacientes en hemodiálisis con fibrilación auricular. Nefrologia. 2018;38:419–426.