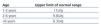Introducción: En una muestra amplia de niños diagnosticados de malformaciones del tracto urinario y/o infección urinaria, hemos calculado los índices de calidad y eficiencia diagnóstica de cinco marcadores funcionales con la intención de comprobar cuáles son los más sensibles para detectar la existencia de una pérdida de parénquima renal. Pacientes y métodos: Estudio retrospectivo transversal en el que se han evaluado las historias clínicas de 179 pacientes en edad pediátrica (91 varones, 88 mujeres). En 102 de ellos (57%), la gammagrafía demostró pérdida de parénquima. Las lesiones morfológicas más frecuentes fueron las cicatrices renales. A todos se les había practicado, al menos, una prueba de concentración realizada con estímulo de desmopresina. Además, se recogieron los resultados de los cocientes albúmina/creatinina y N-acetilglucosaminidasa (NAG)/creatinina, el filtrado glomerular renal (FGR) y el volumen urinario. Resultados: Distribuidos los pacientes según la normalidad o anormalidad de la gammagrafía, se observaron diferencias estadísticamente significativas entre ambos grupos en cuanto a la osmolalidad urinaria máxima y el FGR. El volumen urinario estaba elevado en el 31,3% de los casos (sensibilidad: 37,9%, especificidad: 81,8%) y en el 24% se comprobó defecto de la capacidad de concentración renal (sensibilidad: 30,4%, especificidad: 84,8%). En el 12,2% de los niños la eliminación urinaria de albúmina estaba incrementada y en el 7,2% lo estaba el cociente NAG/creatinina. El FGR estaba reducido únicamente en el 5,7% de los pacientes. Estos dos últimos marcadores fueron los menos sensibles pero los más específicos para detectar pérdida de parénquima renal (100%). Conclusiones: En nuestro estudio, las pruebas funcionales más sensibles para detectar pérdida de parénquima fueron las dos que estudian el manejo renal del agua, volumen urinario y osmolalidad urinaria máxima. Ambas mostraron, además, una especificidad superior al 80%. No obstante, la especificidad fue máxima para el cociente NAG/creatinina y el FGR que, a su vez, fueron las pruebas menos sensibles. Un FGR normal no indica necesariamente una función renal normal.
Introduction: We analysed a large sample of children diagnosed with urinary tract malformations and/or infections and calculated diagnostic efficiency and quality indexes for five different functional markers, with the goal of testing which is the most sensitive for detecting a loss of renal parenchyma. Patients and method: Ours was a cross-sectional retrospective study in which the clinical histories of 179 paediatric patients (91 male and 88 female) were evaluated. In 102 of these patients (57%), a scintigraphy revealed loss of parenchyma. The most commonly observed morphological type of damage was renal scarring. All patients had undergone at least one desmopressin urine concentration test. We also analysed albumin/creatinine and N-acetyl-glucosaminidase (NAG)/creatinine ratios, glomerular filtration rate (GFR), and urine volume. Results: By distributing patients according to normal/abnormal scintigraphy, we observed statistically significant differences between the two groups in maximum urine osmolality and GFR. Urine volume was elevated in 31.3% of cases (sensitivity: 37.9%; specificity: 81.8%) and 24% had a defect in renal concentrating ability (sensitivity: 30.4%; specificity: 84.8%). Urinary albumin excretion was high in 12.2% of patients, and 7.2% had a high NAG/creatinine ratio. GFR was low in only 5.7% of patients. These last two markers were the least sensitive but most specific for detecting a loss of renal parenchyma (100%). Conclusions: In our study, the most sensitive functional tests for detecting the loss of renal parenchyma were the two that take into account the ability of the kidney to manage water, i.e. urine volume and maximum urine osmolality. These two tests had specificity >80%. However, the maximum specificity was obtained by the NAG/creatinine ratio and GFR, which were, conversely, the least sensitive tests. A normal GFR does not necessarily show normal renal function.
INTRODUCTION
Congenital abnormalities of the kidney and urinary tract (CAKUT) are the most common cause of chronic kidney disease in children.1,2 This aetiological association is due to a prenatal reduction in nephrons in some cases and secondary formation of renal scarring when patients suffer from one or more episodes of acute pyelonephritis.3-5 Renal nephropathy is the result of acute pyelonephritis in 25%-57% of cases.6,7
Some malformations are apparently “benign” in the sense that the renal parenchyma remains intact, as occurs in horseshoe kidney, bifid pelvicalyceal system, and crossed-fused renal ectopia, in which renal function is maintained. One peculiar circumstance arises when complete loss of nephrons occurs in one kidney, as is the case in renal agenesis and multicystic dysplastic kidney. In these situations, remaining nephrons progressively undergo a process of hypertrophy starting at birth, which in most cases leads to normal renal function after a certain period of time.8-10 The different types of CAKUT have a different impact on renal function, therefore, determining sensitive markers could be very useful in daily practice and avoid more invasive morphological tests.
In a large sample of children diagnosed with CAKUT and/or urinary tract infection, we calculated diagnostic efficiency and quality indexes for five markers of renal function, with the goal of determining which is the most sensitive for detecting a loss of parenchyma
PATIENTS AND METHOD
Ours was a retrospective, cross-sectional study in which we evaluated the clinical histories t 179 paediatric patients (91 male and 88 female) who were referred to our hospital with a diagnosis of CAKUT and/or one or more urinary tract infections. The mean patient age was 5.84±3.81 years (range: 1-16 years).
The inclusion criteria used were patients older than one year, having undergone at least a dimercaptosuccinic acid (DMSA) scintigraphy and a urine osmolality test. Whenever possible, we recorded the values for albumin/creatinine and N-acetyl-glucosaminidase (NAG)/creatinine ratios from first morning urine samples (n=172 and n=83, respectively). We recorded plasma creatinine values and height in cm in order to determine glomerular filtration rate (GFR) by the Schwartz formula (n=122).11,12 Using plasma and urine creatinine values, we also calculated urine volume adjusted for 100ml GFR (n=99). This value was obtained using the following formula: plasma creatinine x 100 / urine creatinine.13
We used the biochemistry parameters corresponding to the measurements taken closest to the date of the scintigraphy. We excluded patients with persistent vesicoureteral reflux and those who had suffered acute pyelonephritis within the past two months. We also excluded scintigraphic studies that were performed during episodes of acute pyelonephritis, using only those results taken 6-9 months afterwards.
Desmopressin urine concentration test
After emptying the bladder, we administered 20µg of desmopressin intranasally, 0.2mg (200µg) orally, or 0.12mg (120µg) of oral desmopressin lyophilisate (MELT) that dissolves immediately in the mouth.14,15 Then took three consecutive samples, at 90 minute intervals if the patient was continent. The resulting value was the maximum urine osmolality obtained.
Laboratory tests
Creatinine was determined by the creatininase method, using a Modular Analytics analyser (Roche/Hitachi, Mannheim, Germany). Urine osmolality was determined by freezing point depression in an Osmo Station OM-6050 osmometer (Menarini Diagnostics, Florence, Italy). Albumin was measured using a nephelometric technique (Array) and NAG activity was determined using an enzymatic colorimetric assay based on the hydrolysis of NAG-dichlorophenol sulfonephthalein (Boehringer Mannheim).
Normal values
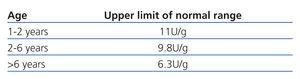
We considered a maximum urine osmolality value of less than 835mOsm/kg to be indicative of defective renal concentration.15,16 Normal values for the two urine ratios mentioned are summarised in Table 1 and Table 2.17-19 Chronic kidney disease was defined as GFR less than 90ml/min/1.73m2. Urine volume was considered elevated if >1.03ml/100ml GFR.13
Statistical methods
In order to examine the distribution of the sample, we used the Kolmogorov-Smirnov test. GFR, which followed a normal distribution, was expressed as mean and standard deviation. All other quantitative variables, which lacked a normal distribution, were expressed as median and interquartile range. Bivariate analyses were used for an initial evaluation of differences. In this manner, we used the Fisher’s exact test to compare frequency between groups of qualitative variables, and the Mann-Whitney U test to compare means of quantitative variables.
Diagnostic efficiency and quality indexes
We calculated sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), likelihood ratio positive (LR+), and likelihood ratio negative (LR-) for the five functional markers analysed. These analyses were carried out using SPSS statistical software (SPSS v. 17.0, SPSS Inc., USA). A P-value <.05 was considered to be statistically significant.
RESULTS
The clinical and radiological diagnoses for all 179 patients are summarised in Table 3. Approximately half of all patients were diagnosed with vesicoureteral reflux (n=98; 54.7%) (4 were level I, 17 were level II, 38 were level III, 33 were level IV, and 6 were level V). The reflux was unilateral in 58 cases and bilateral in 40. In 9 of the 98 cases, the reflux was secondary to posterior urethral valve or neurogenic bladder.
In 77 patients (43%), the scintigraphy failed to demonstrate a loss of parenchyma. In this sub-group, both patients with normal results and those with pyelectasis and horseshoe kidney were included. In the remaining 102 patients (57%), the scintigraphy revealed a loss of renal parenchyma (Table 4). The most common morphological lesion observed was renal scarring.
We observed an impaired renal concentration capacity in 43/179 cases (24%). The urine albumin excretion was elevated in 21/172 cases (12.2%), and the NAG/creatinine ratio in 6/83 (7.2%). In 7/122 (5.7%), GFR was low and urine volume was elevated in 31/99 cases (31.3%).
Analysing our patients based on normal/abnormal scintigraphy results, we observed statistically significant differences between the two groups in maximum urine osmolality and GFR (Table 5).
Tables 6-10 display the comparison of frequencies between normal/abnormal scintigraphy results and normal/abnormal maximum urine osmolality, urine albumin and NAG excretion, GFR, and urine volume, respectively.
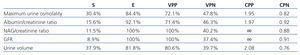
Table 11 shows the values for sensitivity, specificity, positive predictive value, negative predictive value, likelihood ratio positive, and likelihood ratio negative for the five different parameters.
In the 39 patients with morphological or functional absence of one kidney, we analysed the frequency of abnormal results for the different parameters analysed. In all patients, GFR and urine NAG excretion were normal, albumin/creatinine ratio was elevated in 6/37 cases (16.2%), urine osmolality was low in 10/39 (25.6%), and urine volume adjusted for 100ml GFR was elevated in 8/24 (33.3%).
When comparing the values for functional markers between children with one unilateral scar (n=28) and those with one morphologically or functionally absent kidney (n=39), we failed to observe statistically significant differences for any of the parameters, except for GFR (154.8±26.9ml/min/1.73m2 vs 135.3±22ml/min/1.73m2; P=.03).
DISCUSSION
Initially, our study demonstrated that in a large number of cases, despite normal GFR values, functional renal damage may be present, which is not detectable if only normal markers of glomerular function are measured. GFR was the least sensitive parameter for detecting a loss of renal parenchyma (Table 11), which implies that renal compensation mechanisms are able to maintain stable GFR vales until very late stages. As such, when GFR was abnormal, this always corresponded to a loss of parenchyma (specificity: 100%).
NAG is an enzyme characteristic of proximal renal tubular cells. Under normal conditions, it appears in small quantities in urine samples. When proximal tubules are damaged, urinary levels of this molecule increase.19,20 The best example of increased urinary excretion of this and other low-molecular-weight proteins is in the administration of aminoglycoside antibiotics.21,22 In our patients, NAG was elevated, except for one case, when the loss of renal parenchyma was so severe that caused chronic kidney disease. Recent studies have confirmed that the latter situation causes progressive proximal tubular damage,23 which appears to be mediated by the constitutive activation of mTOR signalling pathway.24 The experimental use of everolimus suppresses the accumulation of alpha-actin in smooth muscle cells, macrophage infiltration, and the expression of kidney injury molecule-1 (Kim-1) in proximal tubules.24 The behaviour of NAG was quite similar to that of GFR, both showed low sensitivity and high specificity, meaning that they are very reliable parameters for detecting renal damage. All patients with abnormal results for these two variables had pathological scintigraphy results, such that the likelihood ratio positive was ∞ for both (Table 11). As a result, these are the last parameters of those analysed to become altered in the presence of a loss of renal parenchyma.
Since the late 1980’s, it has been established that albumin appears in small quantities in urine samples in initial stages of diabetic nephropathy and other clinical situations that involve glomerular hyperfiltration.25-27 It is also considered to be an early marker for cardiovascular disease.28,29 Increases in albuminuria have also been described in patients with vesicoureteral reflux,30,31 which led us to exclude these patients from our study. Albuminuria was slightly more sensitive than the previous two parameters (15.6%) and somewhat less specific (92.1%). Albuminuria was normal in 84.4% of patients with a loss of renal parenchyma (Table 7), suggesting that no hyperfiltration existed in remaining nephrons, or alternatively, that an improved glomerularr capacity had developed to protect against excessive glomerular pressure at a paediatric age. A third possible explanation is that the excess filtered albumin may have been reabsorbed in the proximal tubules.32,33
Water management by the kidneys was analysed by determining renal urine concentration and urine volume adjusted for 100ml GFR. Urine concentration is the result of a complex glomerulo-tubular mechanism that culminates in arginine-vasopressin (ADH)-mediated stimulation of aquaporins, the response to which is the reabsorption of water in the renal collecting tubule. When any of the different mechanisms involved (tubular reabsorption of water and solutes in the different segments of the nephron, counter-current mechanism, medullary hyper-osmolality, action of ADH) are altered, the capacity of the kidney to properly concentrate urine decreases, which is associated with polyuria. However, this may not be clinically detectable in mild cases. All chronic kidney disease patients in our study had a reduced maximum urine osmolality (range: 238-518mOsm/kg) and polyuria (range: 1.46-5.48ml/100ml GFR), which has also been described in previous studies.13
Urine volume is closely related with glomerulo-tubular function. It has been established that 99% of the fluid content in glomerular ultrafiltrate is reabsorbed along the renal tubules. Urine volume adjusted for 100ml GFR is rarely used in daily clinical practice. Our results show how useful this simple calculation can be, as it was the most sensitive marker for detecting a loss of renal parenchyma, even slightly superior to maximum urine osmolality. In other words, in patients with a loss of parenchyma, the parameters that examine how kidneys manage water are the first to be affected, and thus the last to return to normal levels.
The capacity for renal parenchyma to go into hypertrophy and compensate for an absence of contralateral renal tissue is very high during paediatric years. As such, agenesis or functional absence of one kidney leads to hypertrophy in the contralateral kidney, which usually results in normal GFR.8-10 However, recent studies have published contrasting results.34,35 In our study, patients with a single functioning kidney had normal values for parameters that are more specific but less sensitive (GFR, urinary NAG excretion), although albuminuria was elevated in 16.2% of cases, and water management by the kidneys was altered in 25.6%-33.3%.
We are aware that one of the limitations of this study was our inability to determine the percentage of parenchymal loss in positive scintigraphy results. Even so, we believe that this limitation is relative, since the evaluation of patients with a loss of renal parenchyma would require a quantification of the number of nephrons and the level of hypertrophy in each one, which would not be feasible in real clinical practice. The complexity of the issue is deepened by the lack of differences in the different parameters measured between children with unilateral renal scarring and those with morphological or functional absence of one kidney, except for GFR. It is difficult to explain why GFR varies between these two groups, although mean and +/-2 SD (standard deviation) values were within normal levels.
To conclude, the most sensitive functional tests for detecting a loss of renal parenchyma were those that examined the ability of the kidney to manage water: urine volume and maximum urine osmolality, both of which had specificity >80%. However, specificity was highest for the NAG/creatinine ratio and GFR, which were also the least sensitive tests. The likelihood ratios positives obtained for all markers were higher than 1, which suggests that all markers are useful for detecting a loss of renal parenchyma in paediatric patients. However, those currently available still have low sensitivity, mainly due to the substantial compensatory capacity of the remaining nephrons. The final conclusion is that normal GFR does not necessarily indicate normal renal function.
Conflicts of interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Table 1. Normal values for albumin/creatinine ratio from the first year of life
Table 2. Normal values for NAG/creatinine ratio from the first year of life
Table 3. Clinical/radiological diagnoses
Table 4. Scintigraphy results
Table 5. Values for the functional parameters according to presence or absence of abnormalities in the scintigraphy
Table 6. Comparison between the results obtained from concentration capacity and scintigraphy studies
Table 7. Comparison between urinary microalbumin excretion and scintigraphy results
Table 8. Comparison between urinary NAG excretion and scintigraphy results
Table 9. Comparison between GFR results and scintigraphy results
Table 10. Comparison between urine volume adjusted for 100ml GFR and scintigraphy results
Table 11. Diagnostic efficiency and quality indexes for the five parameters analysed