Kidney transplant is the treatment of choice for chronic kidney disease. Cardiovascular disease, infections, and postransplant de novo neoplasms are the main causes of death in transplant patients. The most frequent kind of post kidney transplant neoplasms are lymphoproliferative processes and cutaneous neoplasms. Another type of neoplasm, that of kidney tumours, also represents approximately 3% of all neoplasms in transplant patients. A review of the kidney transplants from our unit performed between July 1985 and October 2012 which presented a mass in the kidney graft was carried out, confirming the diagnosis by taking a biopsy of the mass. In all the cases, the underlying pathology, kidney function and immunosuppressive treatment were analysed. This article aims to give importance to monitoring and management of the appearance of possible tumour masses in kidney transplants.
El trasplante renal es el tratamiento de elección de la enfermedad renal crónica. La enfermedad cardiovascular, las infecciones, así como las neoplasias de novo postrasplante renal son las principales causas de mortalidad de los pacientes trasplantados. Las neoplasias más frecuentes en el postrasplante renal son los procesos linfoproliferativos y las neoplasias cutáneas. Otro tipo de neoplasias, como son los tumores renales, también representan aproximadamente el 3 % de todas las neoplasias de los trasplantados. Se ha realizado una revisión de los trasplantados renales de nuestra unidad entre julio de 1985 y octubre de 2012 que han presentado una masa a nivel del injerto renal, confirmando el diagnóstico por biopsia de la masa. Se ha analizado en todos los casos la patología de base, la función renal y el tratamiento inmunosupresor. Este artículo quiere dar importancia a la monitorización de la aparición de posibles masas tumorales en el injerto renal y su manejo.
INTRODUCTION
Kidney transplant is the treatment of choice for end-stage chronic kidney disease as well as the only alternative to renal replacement treatment with dialysis. This fact does not ignore the frequent complications, both medical and surgical that are observed in the short and long term. Despite these complications, patient survival and quality of life have been shown to be greater after transplantation than staying on dialysis. Cardiovascular disease, infections and de novo neoplasms after transplantation are the main causes of death in transplant patients1.
The increasing age of the recipients, the graft’s greater life expectancy and the most powerful immunosuppression patterns make cancer one of the most serious concerns that could compromise the graft’s survival and the the transplant patient’s life.
The most common neoplasms in transplant patients are lymphoproliferative neoplasms and cutaneous neoplasms, which are up to 100 times more frequent than in the general population2. The development of neoplasms has been considered the most common complication in long-term immunosupression treatment3. There are other particular factors that increase the risk of neoplams, such as infections by oncogenic viruses or, in patients with end-stage kidney disease, cystic degeneration acquired from atrophic kidneys3.
In the case of kidney neoplasms, they represent aproximately 3% of all adult neoplasms4, with an incidence rate of 10.02 cases in every 100,000 people per year (0.01%) according to data from the epidemiological study carried out by the Spanish Association of Urology. Of these, approximately 80% are clear cell carcinomas, while only 10-15% are papillary cell tumours5. Greater incidences of this cancer have not been observed in transplant patients in general. However, incidences of neoplasm on native kidneys represents up to 5% in kidney transplant patients6. The mentioned cystic degeneration of the remaining atrophic kidneys after starting dialysis or transplant has been attributed to causing their neoplastic degeneration. Despite this data, in the latest review of the European Association of Urology’s clinical guidelines, cystic kidney degeneration is not included as an aetiological factor4, possibly because the increase in risk is lower in non-transplant patients on dialysis than those who have already received a kidney graft and are on immunosuppression treatment.
Furthermore, a kidney tumour detected on a kidney graft is a very rare entity, only isolated cases and very few series are found in medical literature. The incidence of this is calculated at 0.5% of all kidney transplant patients7 and the latency from the transplant up until its detection is usually a number of years (10-21 years)7,8. The importance of this entity resides in the neoplasm falling on a heterologous organ, normally functioning, which is substituting a prior deficit in the patient. Treatment of these cases is not standardised, but whenever possible, treatments aimed at preserving kidney function should be prioritised, whether they are partial nephrectomies or ablative treatments, as the nephrectomy will inevitably lead to dialysis. Both the size and location of the tumour as well as the kidney function of the graft and the patient’s characteristics will play a part in the decision for appropriate treatment.
MATERIAL AND METHOD
Between July 1985 and October 2012, 813 kidney transplants have been carried out in our centre, 68 of which were transplants from a living donor. After the transplantation, the recipients started a classic regimen of triple immunosuppression therapy with corticoids, calcineurin inhibitors (ciclosporin or tacrolimus) and antiproliferative drugs (azathioprine or mycophenolate). This therapy could be varied depending on rejection episodes or the recipient’s hypersensitisation.
In the cases in which a mass was detected on the kidney graft (n=7) during the post-transplant follow up, a histological confirmation of the neoplasm was performed by a percutaneous biopsy (Trucut 16 G). All the graft’s lesions were ruled out from the study when their malignancy was not confirmed by biopsy or subsequent clinical monitoring. Four cases of tumours in the kidney graft (0.5%) in another four transplant patients have been diagnosed during follow up. None of them showed symptoms and all had a functioning kidney; the tumours were detected by chance in an ultrasound scan. After the suspected diagnosis by ultrasound, a computed axial tomography (CAT scan) on the abdomen and pelvis was performed in all cases as well as the mentioned needle biopsy and a study on the tumour’s stages with a simple chest X-ray, a complete analysis and a bone scan.
RESULTS
The kidney tumours were diagnosed at 10.1 years after the kidney transplant (range: 1-17 years) (median: 13 years), and the average size of the tumour was 32mm (range: 17-50mm). The diagnosed patients followed a tumour extension study, physical examination and the function of the graft’s prognosis was individually analysed.
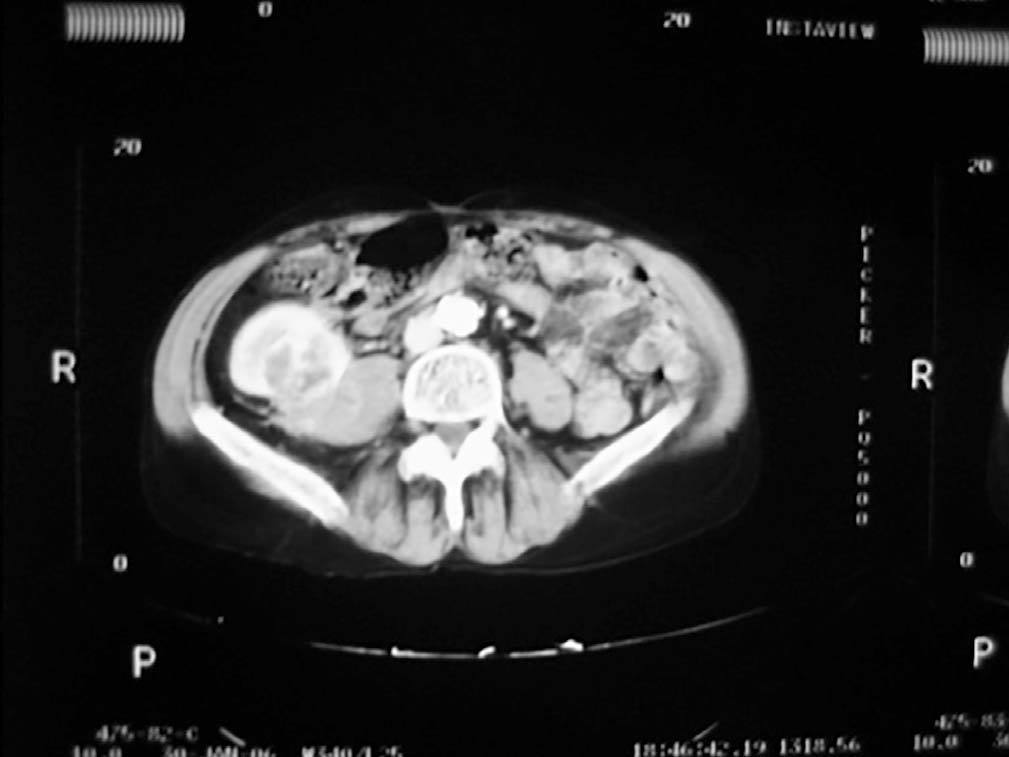
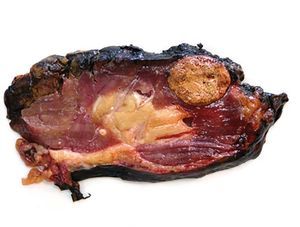
Case 1. A 56-year-old male. He started haemodialysis in 1990 due to hypertensive nephropathy and, in 1992, he was subjected to a kidney transplant from a cadaveric donor without complications. In 2006, without presenting symptoms and due to a deterioration in renal function, a mass with a maximum diameter of 5cm was detected by ultrasound in the upper half of the graft. A percutaneous biopsy was then carried out on the mass which confirmed the presence of renal cell carcinoma. An extension study was carried out with a CAT scan (Figure 1) nuclear magnetic resonance (NMR), which did not show local-regional extension, and an extracapsular transplantectomy with lymphadenectomy. The result in the definitive anatomical pathology was sarcomatoid variant of clear cell carcinomas of 5cm infultrating the renal capsule and surrounding tissue without vein infultration or resected lymph nodes (pT3aN0). After 6.5 years of follow-up, the patient is free from disease. The source of the tumour in the cells of the patient himself was shown in the genetic study with micro-arrays of DNA. The presence of neoplasm in the native kidneys was ruled out and therefore so was the metastatis hypothesis arising from these.
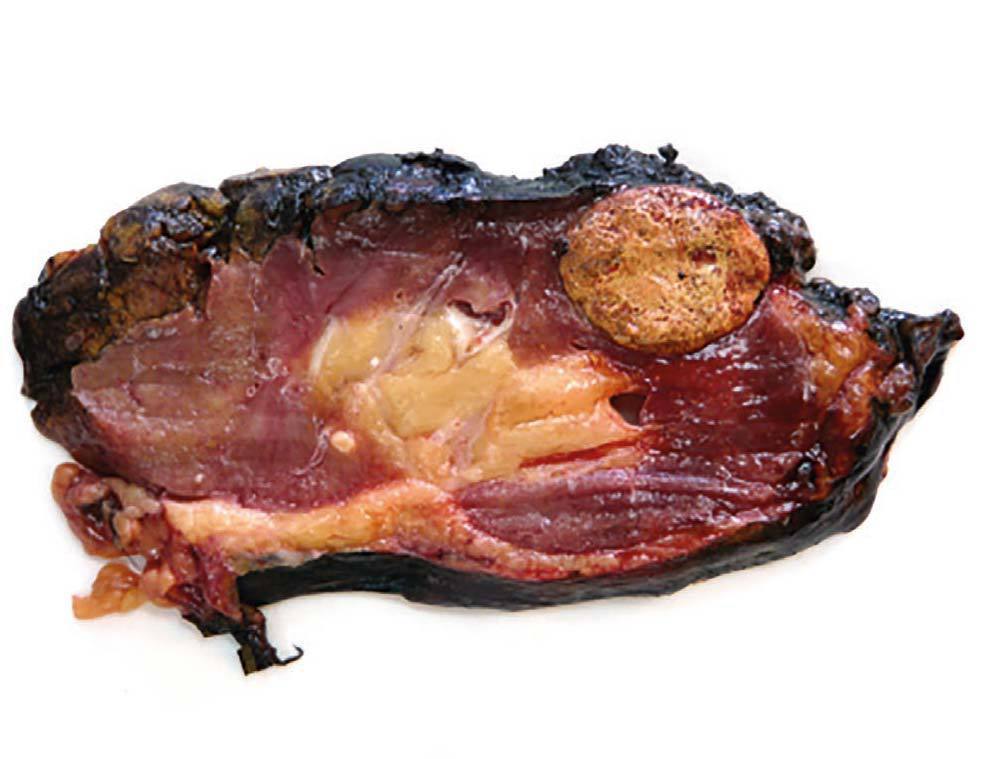
Case 2. The patient was a 32-year-old female, with chronic kidney failure secondary to IgA nephropathy, who received a transplant from a cadaveric donor (male) at the age of 21. Due to progressive deterioration of renal function with creatinine of 3.5mg/dl and glomerular filtration rate estimated at 16ml/min/1.73m2 an ultrasound scan was requested which shows a solid nodule of 32mm in the upper third of the graft. The biopsy conducted confirmed the presence of renal cell carcinoma. The physical examination was normal, the CAT scan did not show signs of tumour extension and the patient showed no symptoms. Given the negative short term prognosis of the graft, it was decided to perform an extracapsular laparoscopic transplantectomy and the patient returned to dialysis after the procedure. The result of the anatomical pathology was papillary renal cell carcinoma of 33mm limited to the kidney with free margins (pT1a) and chronic severe glomerulonephritis in the rest of the kidney (figure 2). After 38 months of follow up, the patient was free from disease. The chromosomal study determined the presence of the Y chromosome in the tumour cells, confirming the donor as the source of the tumour. The other recipient from the same donor was also studied by means of an abdominal ultrasound scan and a CAT scan, without revealing new formations.
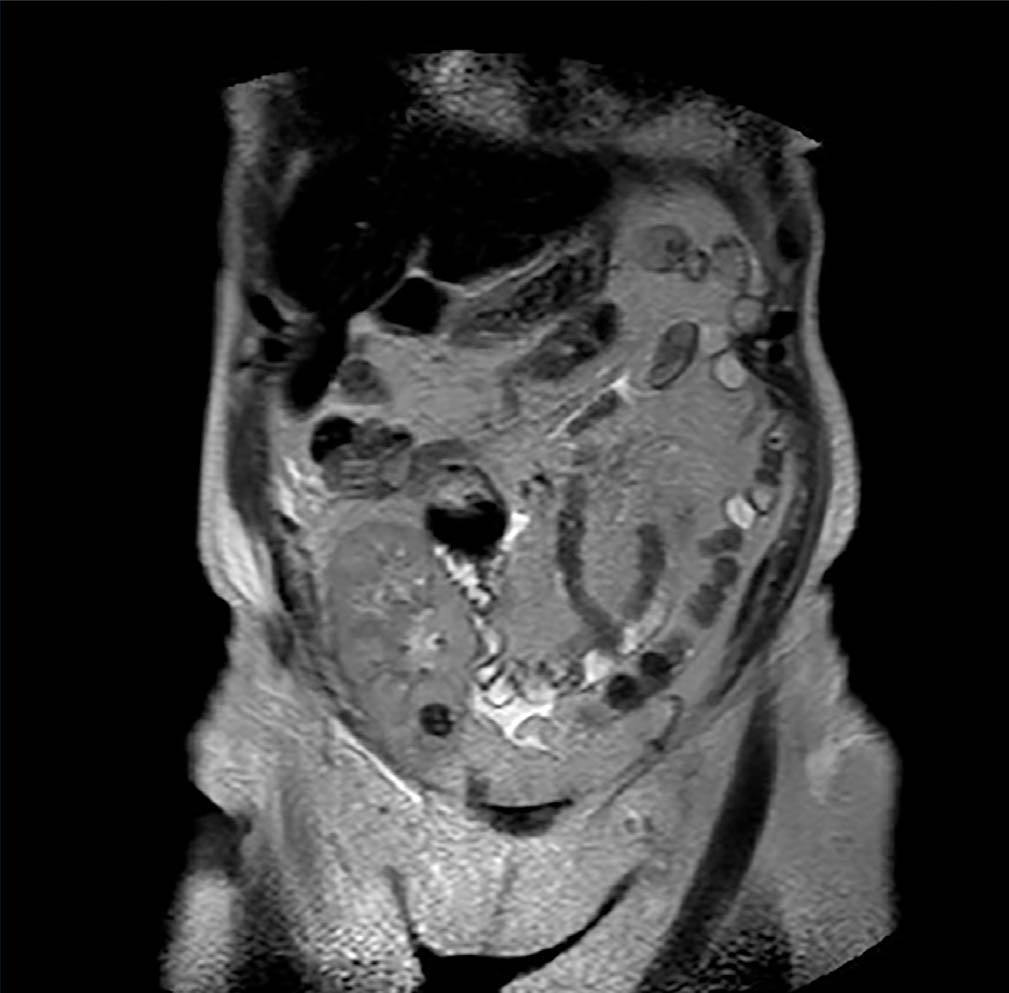
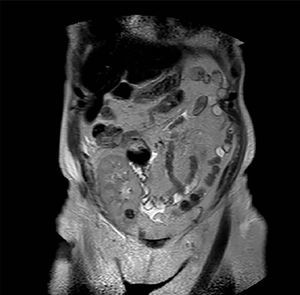
Case 3. Woman of 68 years of age with diabetic nephropathy, on dialysis since 2003. In January 2010 she received a kidney graft from a deceased donor, without postoperative complications and with immediate functioning of the graft. The same donor’s contralateral kidney was rejected for transplant when extracting it due to presenting a biopsy with severe interstitial fibrosis and glomerulosclerosis and multiple minute cortical lesions compatible with tubular adenomas, while the biopsy and the macroscopic aspect of the implanted graft were satisfactory. During a routine check-up after 12 months a hyperechogenic nodule was detected by ultrasound in the graft’s upper half with a maximum diameter of 12mm. It was intrarenal and was not seen in previous examinations. The existence of said nodulation was confirmed by NMR (Figure 3) and it was decided to perform a renal biopsy that confirmed the presence of cells compatible with type I papillary cell carcinoma. Renal function was strictly normal and the patient decided to follow periodic ultrasound check-ups and not to take any ablative steps in the first instance, due to the optimal kidney function and the dificulty in accessing the tumour for conservative treatment. Immunosuppression treatment had been started with prednisone, tacrolimus and mycophenolic acid, and the immunosuppression was then changed, substituting the tacrolimus with everolimus after diagnosing the kidney tumour. After 22 months of monitoring the patient she shows no symptoms, with creatinine levels at 1.15mg/dl and changes in the tumour’s size have not been shown in the subsequent ultrasound scans The recipient of the liver from the same donor was also studied, and was subjected to an imaging study without finding suspected masses in the graft.
Case 4. The patient was a 72-year-old female, and a carrier of IgA nephropathy who started haemodialysis in 1989. In 1994 she received a kidney transplant from a deceased male donor. The subsequent check-ups have been correct and in the last few years she has had kidney function with levels of creatinine at 1.9mg/dl, MDRD 23. In July 2011, a follow-up ultrasound scan was performed in which a solid 20mm nodule was shown in the upper half of the graft. A biopsy of the nodule was carried out, which was positive for renal cell carcinoma, as well as a biopsy of the kidney free from tumours which confirmed relapse of IgA nephropathy. Given the patient’s age and quality of life as well as the size and location of the tumour, it was decided to perform a partial laparoscopic nephrectomy without ischemia. The analysis of the anatomical pathology of the tumour was papillary renal cell carcinoma with a maximum diameter of 22mm, encapsulated and with margins free from neoplasm (pT1a). No change in the immunosuppression treatment was made, given that the glomerular filtratation rate was <30ml/min/1.73m of body surface, which at that moment was only prednisone and cyclosporin. After 14 months of follow up, the patient maintains the same kidney function as before the intervention. In the chromosomal study of the tumour the Y chromosome was detected in the tumour cell which showed the donor was the source. The pair that received the other kidney from the same donor was equally studied, without showing lesions on their graft.
The average follow-up time for patients was 37.5 months (14-76). median of 30 months, without showing relapses in any of the patient’s cases nor did the size of the tumour increase in the patient who it was decided to follow.
DISCUSSION
The appearance of a tumour in a kidney graft is uncommon. An incidence of 0.5% has been calculated in the transplant patients, and this figure is quite consistent in the few series published7-10. This incidence is much greater than that of kidney tumours in the general population, but it is less than the neoplasms in the native kidney in transplant patients8. The specific reason for the increase of the incidence of tumours in transplanted kidneys in regard to the general population is unknown, but the chronic immunosuppression that the recipient is subjected to along with the greater monitoring (and as a consequence, earlier detection of small sized lesions) could be the cause. Despite the fact that the incidence is low, it could be expected that in the next few decades it will rise due to the increase of the grafts’ survival and the progressive rise in the age of the donors who we have been working with in recent years.
The latency period between the transplant and the diagnosis of the tumour is usually long, with periods generally greater than 10 years, and even 20 years after the transplantation in one case described11. However, there are some series and isolated cases of diagnoses before 24 months after the transplant12, which would corroborate the hypothesis of the kidney transplant with minute or invisible lesions at the time of extraction or implantation, but these cases make up a small proportion. Indeed, in the series we are presenting, one of the cases was diagnosed 12 months after the implant. In this case in particular, the contralateral kidney was rejected both because of the presence of multiple minute macroscopic lesions visible on the kidney surface and because of the finding of tubular adenomas in the biopsy, although no malignant cells were shown in such graft. In the cases in which a genetic study has been carried out, it has been shown that the tumour making cells in the graft come from the donor themselves, i.e. the neoplastic degeneration years after the implantation of the graft’s cells is admitted. The theory of the degeneration of the transplanted kidney cells themselves, encouraged by a prolonged immunosuppression environment is the most plausible factor responsible and would explain the increase in frequency in relation to the general population. However, in one case in our series (and unique in medical literature) the source of the tumour was found in the recipient’s cells13, thus attributing the source to the existence of circulating stem cells that are implanted in the graft and degenerate.
The treatment of a kidney tumour found in a graft is not standardised10, and the size of the tumour, its location and the graft’s function prognosis will influence the decision to be made. In the past, there was a tendency to completely remove the graft when dealing with an immunodeficient patient with a neoplasm, but this attitude takes the patient back to dialysis, which is what was hoped to be avoided with the transplant. The current trend is to consider partial surgery as necessary for treating a patient with one kidney. This is because despite the immunosuppression, the recurrence and progression of this tumour type has not been shown as greater than that of the general population without transplants, nor have relapses been reported after conservative treatments in spite of being carried out on masses of up to 6cm14. Partial nephrectomies are normally performed by open surgery, although in 2009 the first laparoscopic partial nephrectomy on a kidney graft was published15. It has been reported, both in series and in isolated cases and in most partial nephrectomy cases that the number of transplantectomies is low: 4 in 17 cases in Pluossard’s series7 and 3 in 8 cases in Leveridge’s series8, as well as a transplantectomy carried out in 1992 for a tumour of 4.5cm in the lower half of the normally functioning graft16, which would have probably been treated with patial surgery today.
In the cases in which partial surgery is chosen, the persistence of the allograft in the recipient obliges the maintaining of immunosuppression treatment after the diagnosis; but, given that prolonged immunosuppression has been related to the appearance of neoplasms, it is recommended to either reduce the immunosuppression or modify the pattern by incorporating drugs that provide protection against rejection and at the same time provide an antiproliferative effect on tumours. In the cases of kidney neoplasm, introducing everolimus as an immunosuppression treatment seems to be the norm17. Its effect as an inhibitor of the action of the T lymphocytes combines the immunosuppression effect with the antiproliferative effect (indicated as second line chemotherapy in advanced renal carcinoma), blocking the growth factor of the vascular endothelium. Not needing to modify the dosis for age or for renal failure makes this drug ideal for the cases of neoplasm in the kidney graft that may need to continue with immunosuppression treatment.
The practically null incidence of neoplasms detected in non-functioning grafts is remarkable. We do not know if this is because it has not been reported in medical literature or because there is a protective factor when removing or decreasing immunosuppression. On reviewing the literature, we find only one case of squamous cell carcinoma in a previously rejected graft18. The periodic follow up with imaging tests to which transplant patents are subjected would explain the early diagnosis of small sized masses, before they have time to grow much, silently. However, this does not explain the small number of tumours in lost grafts when the transplantectomy after the loss of the organ is not the norm, most centres opt to leave the graft if it does not provoke clinical intolerance19.
In conclusion, neoplasms in kidney grafts are uncommon, although they have an incidence greater than that of kidney tumours in the general population and they generally appear years after the transplantation. In these cases, a confirmatory biopsy must be performed in all cases before deciding on the treatment to be followed. The periodic follow up that these patients are subjected to allow these masses to be detected in the early stages and the kidney preservation therapies (partial surgery or ablative techniques) might be considered as a first treatment option in order to thus preserve the function of the graft and avoid dialysis. At the time of diagnosis, it is recommended to modify the immunosuppression pattern by introducing everolimus to the treatment for its double immunosuppression and tumour prevention (antiproliferative) action.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Sarcomatoid variant of renal cell carinoma
Figure 2. Papillary renal cell carcinoma
Figure 3. Type I papillary cell carcinoma