Gram-negative enterobacteria resistant to carbapenem pose a problem, as this antibiotic is usually used for infections caused by Enterobacteriaceae producing extended spectrum β-lactamases.1Klebsiella pneumoniae carbapenemase (KPC) enzymes are the most common class A carbapenemases.2 We have reported the first cases of blaKPC-2-producing K. pneumoniae (KPC-Kp) in kidney transplant patients in our center.3 We describe the treatment and outcomes of 13 new patients.
This retrospective cohort study was conducted at Hospital Alemán, Buenos Aires, Argentina. In the study period (1/2011–8/2013), 93 renal transplants were performed. Disk diffusion antimicrobial susceptibility tests were performed according to the Clinical and Laboratory Standards Institute guidelines. KPC was screened with double-disk diffusion synergy tests; a disk containing 300-μg phenyl boronic acid and a disk containing 10-μg imipenem, 10-μg meropenem, or 10-μg ertapenem. The modified phenotypic Hodge test was performed for isolates exhibiting reduced susceptibility to imipenem or meropenem on the disk diffusion test. blaKPC-2 was confirmed by PCR with primers KPC-F (5′-ATGTCACTGTATCGCCGTCT-3′) and KPC-R (5′-TTTTCAGAGCCTTACTGCCC-3′), heat-extracted DNA as template. Molecular typing was performed using pulsed-field gel electrophoresis, XbaI restriction enzyme; with strain sequence type 258 clone as reference. Tested antibiotics: ampicillin, ampicillin/sulbactam, amoxicillin/clavulanic acid, cephalothin, piperacillin/tazobactam, cefotaxime, ceftazidime, imipenem, meropenem, ertapenem, gentamicin, amikacin, ciprofloxacin, doxycycline, trimethoprim/sulfamethoxazole, nitrofurantoin, and fosfomycin. Tigecycline susceptibility was tested by disk diffusion (susceptible ≥19mm, intermediate 15–18mm, and resistant ≤14mm). The isolates intermediate or resistant on disk testing were confirmed using MIC plates. Polymyxin B susceptibility was determined with Etest® (bioMérieux, Marcy l’Etoile).
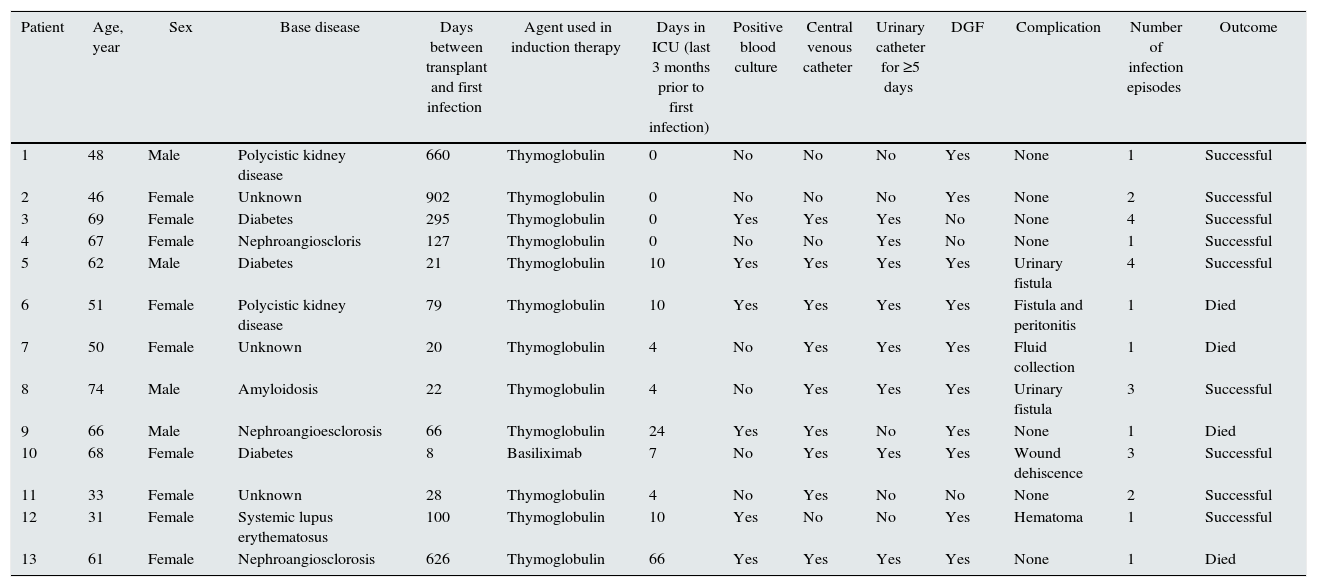
Twenty-five KPC-Kp infectious episodes were documented in 13 renal transplant recipients. Mean age was 55.84±13.84 years, 9 (62%) patients were female, and the most frequent primary kidney diseases were diabetes, polycystic kidney disease. All patients received antibiotic prophylactic therapy and induction therapy; 12/13 (92.3%) with thymoglobulin and 1/13 with basiliximab. Nine (69%) patients had been in the intensive care unit (ICU) within 30 days before their first infection, median time of stay was 4 days (0–66 days). Six (46%) patients showed surgical complications, 10/13 (77%) patients had delayed graft function, 9/13 (69%) patients had a central venous catheter and 8/13 (61%) had a urinary catheter for ≥5 days within 3 months before infection. The median time between transplant and the first infection was 79 (8–902) days. Six (46%) patients had more than two infections, totaling 25 infectious episodes (Table 1).
Patients¿ characteristics and outcomes.
| Patient | Age, year | Sex | Base disease | Days between transplant and first infection | Agent used in induction therapy | Days in ICU (last 3 months prior to first infection) | Positive blood culture | Central venous catheter | Urinary catheter for ≥5 days | DGF | Complication | Number of infection episodes | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | Male | Polycistic kidney disease | 660 | Thymoglobulin | 0 | No | No | No | Yes | None | 1 | Successful |
| 2 | 46 | Female | Unknown | 902 | Thymoglobulin | 0 | No | No | No | Yes | None | 2 | Successful |
| 3 | 69 | Female | Diabetes | 295 | Thymoglobulin | 0 | Yes | Yes | Yes | No | None | 4 | Successful |
| 4 | 67 | Female | Nephroangioscloris | 127 | Thymoglobulin | 0 | No | No | Yes | No | None | 1 | Successful |
| 5 | 62 | Male | Diabetes | 21 | Thymoglobulin | 10 | Yes | Yes | Yes | Yes | Urinary fistula | 4 | Successful |
| 6 | 51 | Female | Polycistic kidney disease | 79 | Thymoglobulin | 10 | Yes | Yes | Yes | Yes | Fistula and peritonitis | 1 | Died |
| 7 | 50 | Female | Unknown | 20 | Thymoglobulin | 4 | No | Yes | Yes | Yes | Fluid collection | 1 | Died |
| 8 | 74 | Male | Amyloidosis | 22 | Thymoglobulin | 4 | No | Yes | Yes | Yes | Urinary fistula | 3 | Successful |
| 9 | 66 | Male | Nephroangioesclorosis | 66 | Thymoglobulin | 24 | Yes | Yes | No | Yes | None | 1 | Died |
| 10 | 68 | Female | Diabetes | 8 | Basiliximab | 7 | No | Yes | Yes | Yes | Wound dehiscence | 3 | Successful |
| 11 | 33 | Female | Unknown | 28 | Thymoglobulin | 4 | No | Yes | No | No | None | 2 | Successful |
| 12 | 31 | Female | Systemic lupus erythematosus | 100 | Thymoglobulin | 10 | Yes | No | No | Yes | Hematoma | 1 | Successful |
| 13 | 61 | Female | Nephroangiosclorosis | 626 | Thymoglobulin | 66 | Yes | Yes | Yes | Yes | None | 1 | Died |
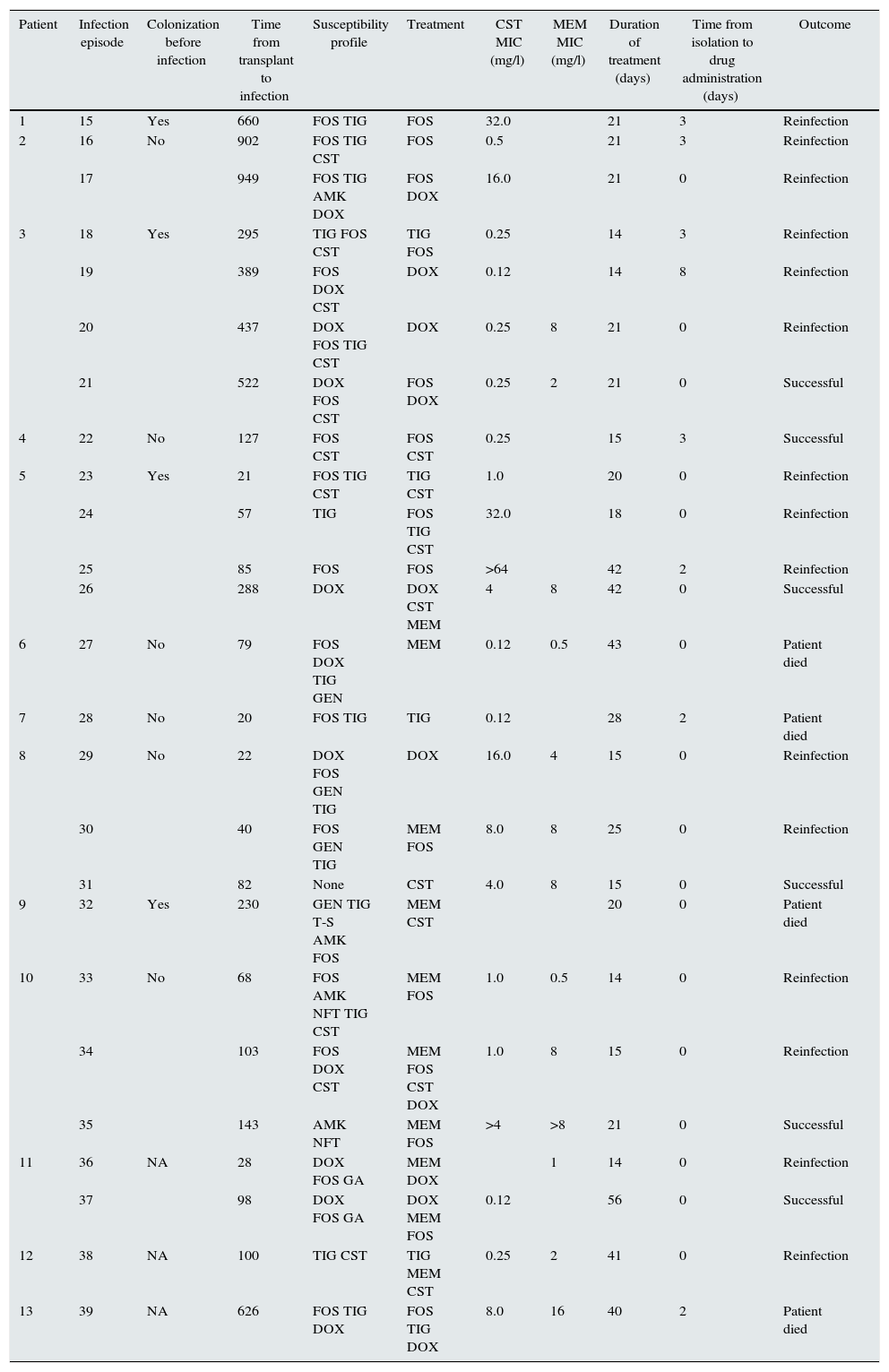
In all episodes, the site of infection was the urinary tract and some antibiotic had been administered within 30 days before diagnosis; however, in 10/25 (40%) episodes, the blood culture showed positive results. The most frequent antibiotics in which bacteria were susceptible were fosfomycin (68%), tigecycline (56%), and colistin (28%). In 1 case, the organism was not susceptible to any tested antibiotic, and in 3/25 (12%) cases, it was susceptible to one drug. Monotherapy was used in 9/25 (36%) episodes; two drugs were used in 10/25 (40%), three in 5/25 (20%), and four in 1/25 (4%) episodes. Table 2 shows the antibiotics used. Tigecycline, alone or combined, resulted in an unsuccessful outcome. The median treatment time was 21 (14–56) days. Four (31%) patients died as a result of infection.
Infection episodes, susceptibility patterns, treatments and outcomes.
| Patient | Infection episode | Colonization before infection | Time from transplant to infection | Susceptibility profile | Treatment | CST MIC (mg/l) | MEM MIC (mg/l) | Duration of treatment (days) | Time from isolation to drug administration (days) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | Yes | 660 | FOS TIG | FOS | 32.0 | 21 | 3 | Reinfection | |
| 2 | 16 | No | 902 | FOS TIG CST | FOS | 0.5 | 21 | 3 | Reinfection | |
| 17 | 949 | FOS TIG AMK DOX | FOS DOX | 16.0 | 21 | 0 | Reinfection | |||
| 3 | 18 | Yes | 295 | TIG FOS CST | TIG FOS | 0.25 | 14 | 3 | Reinfection | |
| 19 | 389 | FOS DOX CST | DOX | 0.12 | 14 | 8 | Reinfection | |||
| 20 | 437 | DOX FOS TIG CST | DOX | 0.25 | 8 | 21 | 0 | Reinfection | ||
| 21 | 522 | DOX FOS CST | FOS DOX | 0.25 | 2 | 21 | 0 | Successful | ||
| 4 | 22 | No | 127 | FOS CST | FOS CST | 0.25 | 15 | 3 | Successful | |
| 5 | 23 | Yes | 21 | FOS TIG CST | TIG CST | 1.0 | 20 | 0 | Reinfection | |
| 24 | 57 | TIG | FOS TIG CST | 32.0 | 18 | 0 | Reinfection | |||
| 25 | 85 | FOS | FOS | >64 | 42 | 2 | Reinfection | |||
| 26 | 288 | DOX | DOX CST MEM | 4 | 8 | 42 | 0 | Successful | ||
| 6 | 27 | No | 79 | FOS DOX TIG GEN | MEM | 0.12 | 0.5 | 43 | 0 | Patient died |
| 7 | 28 | No | 20 | FOS TIG | TIG | 0.12 | 28 | 2 | Patient died | |
| 8 | 29 | No | 22 | DOX FOS GEN TIG | DOX | 16.0 | 4 | 15 | 0 | Reinfection |
| 30 | 40 | FOS GEN TIG | MEM FOS | 8.0 | 8 | 25 | 0 | Reinfection | ||
| 31 | 82 | None | CST | 4.0 | 8 | 15 | 0 | Successful | ||
| 9 | 32 | Yes | 230 | GEN TIG T-S AMK FOS | MEM CST | 20 | 0 | Patient died | ||
| 10 | 33 | No | 68 | FOS AMK NFT TIG CST | MEM FOS | 1.0 | 0.5 | 14 | 0 | Reinfection |
| 34 | 103 | FOS DOX CST | MEM FOS CST DOX | 1.0 | 8 | 15 | 0 | Reinfection | ||
| 35 | 143 | AMK NFT | MEM FOS | >4 | >8 | 21 | 0 | Successful | ||
| 11 | 36 | NA | 28 | DOX FOS GA | MEM DOX | 1 | 14 | 0 | Reinfection | |
| 37 | 98 | DOX FOS GA | DOX MEM FOS | 0.12 | 56 | 0 | Successful | |||
| 12 | 38 | NA | 100 | TIG CST | TIG MEM CST | 0.25 | 2 | 41 | 0 | Reinfection |
| 13 | 39 | NA | 626 | FOS TIG DOX | FOS TIG DOX | 8.0 | 16 | 40 | 2 | Patient died |
Discussion: Transplant recipients share risk factors for infections with resistant bacteria.4 In a report describing an outbreak of KPC-Kp in transplant patients, infection rates were 17%, 13%, and 26% in heart, liver, and kidney transplant recipients, respectively.5 In our cases, the infection rate was 16% and strain sequence type 258.
The best treatment for KPC has not been established, and we could not identify a successful treatment. Before obtaining susceptibility, empirical treatment was administered; a frequent reported combination therapy is tigecycline plus colistin, but the empirical use of colistin could contribute to selection of resistant strains.6 We avoided nephrotoxic agents, and took into consideration the drug concentration at the site of infection and potential adverse events. Carbapenem monotherapy has a higher rate of treatment failure compared to combination therapy; moreover, only the combination of meropenem, colistin, and tigecycline is associated with improved survival in patients with blood infections.6 Colistine was successful when the isolate showed resistance to all tested antibiotics. The use of a triple-drug regimen including tigecycline, colistin, and meropenem was linked to a reduced risk of death.7
To prevent the spread of bacteria, measures such as perirectal surveillance swabs, susceptibility tests, use of gowns and gloves, and strict hand hygiene were followed. Also, recipients of kidneys from living donors were transferred from the operating room to a non-intensive setting, or underwent a reduced stay in the ICU. Despite its limitations, this report shows that UTIs caused by KPC-Kp pose a threat to kidney transplant patients.
The molecular methods were performed by Microbiology Chair of Faculty of Pharmacy and Biochemistry, Universidad de Buenos Aires, Argentina. We are indebted to Liliana Fernández Canigia MD, from Microbiology at Hospital Alemán, Buenos Aires, for providing K. pneumoniae strains.