In post-dilution haemodiafiltration only synthetic membranes have been used to date. Asymmetric cellulose triacetate (ATA™) is now available, whose characteristics are suitable for this technique.
ObjectivesTo describe the in vivo performance and behaviour of this membrane, to identify its depurative effectiveness, use in clinical practice and its biocompatibility, both acute and after one month of treatment.
MethodsObservational prospective study of 23 patients who were dialysed for 4 weeks using an ATA™ membrane and who maintained their prior regimen.
ResultsA total of 287 sessions were performed and 264 complete sessions were collected. With an effective time of 243.7 (17.6) min and a mean blood flow of 371.7 (23) ml/min, an average Kt of 56.3 (5.3) l was observed, as well as a convection volume of 27.1 (4.2) l, a filtration fraction of 29.9 (3.7) %, a urea reduction ratio (RR) of 81 (5.2) %, a creatinine RR of 74.7 (4.6) %, a β2-microglobulin RR of 76.5 (4.8) % and a retinol binding protein RR of 18.6 (7.6) %. There were no technical problems or alarms. Changing the heparin dosage was not necessary. No increases in C3a or C5a concentrations or leukopenia were observed in the first 30min of the session. Neither the monocyte subpopulations nor IL-β1 or IL-6 were significantly altered after one month of treatment.
ConclusionsThe new ATA™ membrane achieves adequate Kt and convection volume, without technical problems and with good biocompatibility and inflammatory profiles. It is therefore a valid option for post-dilution haemodiafiltration, particularly in patients allergic to synthetic membranes.
En la hemodiafiltración posdilucional se han usado solo membranas sintéticas. Ahora contamos con un triacetato de celulosa asimétrico (ATA®) cuyas características lo hacen apto para esta técnica.
ObjetivosDescribir las prestaciones y el comportamiento in vivo de esta membrana estudiando la eficacia depurativa y el uso clínico, además de su biocompatibilidad aguda tras un mes de tratamiento.
MétodosEstudio prospectivo observacional en el que se incluyeron 23 pacientes que se dializaron durante 4 semanas con ATA® manteniendo su pauta previa.
ResultadosSe realizaron 287 sesiones y se recogieron 264 sesiones completas. Con un tiempo efectivo de 243,7 (17,6) min y un flujo medio de sangre de 371,7 (23) ml/min, se obtuvo un Kt medio de 56,3 (5,3) l, un volumen convectivo de 27,1 (4,2) l, con una fracción de filtración del 29,9 (3,7) %, un porcentaje de reducción (RR) de urea de 81 (5,2) %, un RR de creatinina de 74,7 (4,6) %, un RR de β2-microglobulina de 76,5 (4,8) % y un RR de proteína transportadora de retinol de 18,6 (7,6) %. No se produjeron problemas técnicos ni alarmas. No fue preciso cambiar la dosificación de heparina. A los 30min de la sesión no se produjo ningún aumento de C3a, C5a ni leucopenia. Tampoco se modificaron de forma significativa las poblaciones monocitarias ni la IL-β1 ni IL-6 tras un mes de tratamiento.
ConclusionesATA® logra un Kt y un volumen convectivo adecuados, sin problemas técnicos y con buen perfil de biocompatibilidad e inflamatorio, lo que lo convierte en una posibilidad más de tratamiento para hemodiafiltración posdilucional, máxime en pacientes alérgicos a membranas sintéticas.
Online haemodiafiltration (OLHDF) is the most complete haemodialysis technique currently available, as it is capable of removing significant quantities of low-, medium- and high-molecular weight uraemic toxins, in direct relation with the convective transport volume obtained.1 Post-dilution OLHDF is the most used form and has proven efficacy. It is known to be a safe technique which improves intradialytic haemodynamic tolerance and increases survival.2–4
OLHDF uses high-flux biocompatible dialysers, providing greater clearance per unit of surface by combining the processes of diffusion and convection. To date, the membranes used in this technique have been synthetic. In the study by Maduell et al.,5 in which multiple dialysers for OLHDF were compared, they considered that cellulose triacetate had a low purification of β2-microglobulin and limited its use in OLHDF due to high transmembrane pressure (TMP). An asymmetric cellulose triacetate membrane (ATA®) was recently marketed in the Solacea® (Nipro) dialyser with a KUF of 72ml/mmHg/h/m2 and configured in order to perform OLHDF. In accordance with the manufacturer's data, the complement activation produced is similar to that of the synthetic membranes, demonstrating its biocompatibility. Its long-term biocompatibility through its effect on monocytes and interleukins (IL) is still to be determined. As yet, there is no publication on the convective volume (Vconv) obtained, the adaptation to different OLHDF systems, the clearance capacity for different types of molecules or biocompatibility in daily clinical practice. The recent description of various cases of hypersensitivity with biocompatible membranes, such as polysulfone,6 in which cellulose triacetate is used as an alternative, has led to even greater interest in the study of this type of membrane.
ObjectiveTo describe the features and the in vivo behaviour of the ATA® membrane in order to identify: its clearance efficacy, biocompatibility and ease of use in clinical practice.
Material and methodsThis was a prospective, observational study in three hospital haemodialysis units (Hospital de La Princesa, Hospital Príncipe de Asturias and Hospital Infanta Leonor, Autonomous Community of Madrid, Spain) in which the normal synthetic dialyser that each patient had for OLHDF was replaced with an ATA® dialyser, with all other parameters remaining the same. The study (LIB 09/2015) was reviewed and approved by the Hospital Universitario Príncipe de Asturias IEC (Alcalá de Henares, Madrid).
Inclusion and exclusion criteriaInclusion criteria: over 18 years of age, having undergone treatment with OLHDF for more than four weeks (with three weekly sessions) and having signed the informed consent form.
Exclusion criteria: pregnancy and illness which means that death is predicted to occur in fewer than four weeks.
Study designEach patient underwent 12 haemodialysis sessions (during one month) with his/her normal regimen of time, bathing, heparin dose, sodium and bicarbonate conductivity and also on his/her normal monitor: 5008-Fresenius® (n=14), AK 200-Gambro® (n=5), Artis-Gambro® (n=3) and the Nikkiso DBB-07 (n=1). All monitors were suitable for conducting OLHDF, although with different automated control systems for convective transport. The Qb used was the maximum facilitated vascular access, without allowing an arterial line the pressure below −220mmHg.
In all cases, the nursing staff connected the automated OLHDF system to the system with which the patient's monitor operated. In the event that the system was Ultracontrol®, the warning systems were set at TMP>300 or pressure system (Psyst)>700mmHg. In the event that these warnings were shown and not resolved, Ultracontrol® would be withdrawn and the control-pressure system would be used, in which the TMP remains at values which ensure a Psyst<700mmHg and an appropriate infusion volume (Vinf).
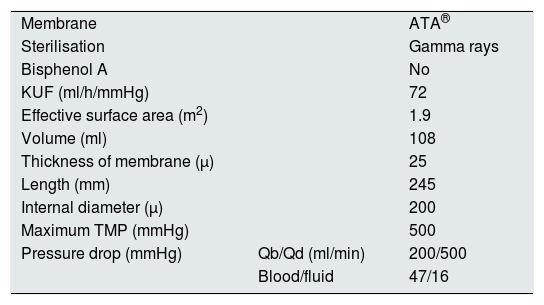
Dialyser featuresThe features of the dialyser are specified in Table 1.
Technical features of the Solacea® 19H dialyser.
| Membrane | ATA® | |
| Sterilisation | Gamma rays | |
| Bisphenol A | No | |
| KUF (ml/h/mmHg) | 72 | |
| Effective surface area (m2) | 1.9 | |
| Volume (ml) | 108 | |
| Thickness of membrane (μ) | 25 | |
| Length (mm) | 245 | |
| Internal diameter (μ) | 200 | |
| Maximum TMP (mmHg) | 500 | |
| Pressure drop (mmHg) | Qb/Qd (ml/min) | 200/500 |
| Blood/fluid | 47/16 |
KUF: ultrafiltration coefficient; Qb: blood flow; Qd: dialysis flow; TMP: transmembrane pressure.
- -
Demographic data: gender, age, underlying disease, time on OLHDF, type of vascular access: fistula or catheter.
- -
General information related to the dialysis procedure: monitor, composition of dialysis fluid (calcium, potassium) sodium and bicarbonate conductivities, dialysis fluid flow (Qd, ml/min), fluid temperature, type and dose of heparin.
- -
In each dialysis session: effective time (min), blood flow (Qb, ml/min), volume ultrafiltrated to achieve dry weight (UF, l/session), Vinf (l/session), infusion rate (Qi, ml/min), Kt (l/session), maximum TMP and maximum Psyst in Ultracontrol® (mmHg) and the technical complications, warnings and system coagulation problems that could occur.
The filtration fraction (FF) was calculated as the percentage of Qi relative to Qb. Following the definition of the EuDial group, the Vconv was defined as the total ultrafiltrate volume, which is the sum of Vinf and UF.7
Blood tests- -
Pre-dialysis samples were obtained on the first and last day to measure monocytes and IL-6 and IL-1β.
- -
On the mid day of the first week, three blood extractions were performed: the first, at the start (CI); the second, after 30min (CM), and the third, at the end of the dialysis session (CP). All from the arterial line: the first directly from the patient, before the connection, and the other two after reduction of Qb to 50ml/min. during one minute.
The following were measured at CI and CP: blood count; proteins and albumin; small molecules (molecular weight less than 500Da): urea (60Da), phosphorus (95Da), creatinine (113Da) and uric acid (168Da); medium molecules (molecular weight greater than 500Da): β2-microglobulin (11,818Da) and myoglobin (17,200Da); and molecules bound to proteins: retinol-binding protein (21,200Da).
Leukocytes, platelets, C3a and C5a were measured to study acute biocompatibility at CI and CM.
Laboratory methodsGeneral biochemical data: blood count, proteins, albumin, urea, phosphorus, creatinine (113Da), uric acid, β2-microglobulin, myoglobin and retinol-binding protein were determined with each hospital's regular analyser.
The determinations of monocytes, C3a, C5a, IL-6 and IL-1β were performed at the Universidad de Alcalá laboratory. C3a and C5a were measured with the ELISA Kit C3a Elabscience (Wuhan, PR China) and ELISA C5a RayBiotech (Norcross, GA, USA). The concentration of IL-6 and IL-1β was measured in frozen serum samples with Abcam kits (Cambridge, United Kingdom). The populations of monocytes were identified by flow cytometry (FACSCalibur™, Becton Dickinson, San Jose, CA, USA).
CalculationsThe percent reduction ratios (RR) were calculated with the formula: RR (%)=[(Cpre−Cpos)/Cpre]×100, where Cpre and Cpos are the concentrations of the compounds analysed pre- and post-dialysis.
For the substances bound to proteins and the β2-microglobulin, the concentrations at the end of the session were corrected for haemoconcentration by a correction factor based on the change in plasma protein concentration:
Correction factor=TPpre/TPpos,8where TPpre and TPpos are the concentrations of total proteins pre- and post-dialysis.
Statistical analysisAll the information was collected in a database file to be analysed using SPPS vs .15. Each value was the mean of the values obtained from the different sessions or blood determinations.
For the statistical analysis, descriptive statistics included means (standard deviation), median with quartiles or percentages, as appropriate. Paired Student's t-test was used to compare paired measurements from continuous variables. p<0.05 was considered to be statistically significant.
ResultsThe study included 23 patients (15 males, 8 females) with a median age of 65 (41–85). The causes of kidney disease were: diabetic nephropathy in seven cases, glomerular disease in six cases, nephroangiosclerosis in two cases, reduced kidney mass in two cases, hepatorenal polycystic disease in two cases, chronic pyelonephritis in one case and unknown origin in three cases. The median time on renal replacement therapy was 98 (17–315) months. Sixteen patients had arteriovenous fistula and seven had a catheter. The mean dry weight was 73.4 (16.7) kg.
A total of 287 sessions were performed, and 264 complete sessions were collected (sessions excluded were those in which the effective time differed by more than 15min from the scheduled time, or if the monitor had been changed). One patient was lost to follow-up due to vascular disease requiring hospital admission .
The results of dialysis parameters and molecule clearance are shown in Tables 2 and 3, respectively. The FF obtained with the different monitors was: 5008® 28.9 (3.9)%, AK 200® 31.8 (1.8)%, Artis® 32.6 (4)% and DBB-07® 27%. There was a negative correlation between the initial Hb and the Vconv (r=−0.5, p>.01) and the Kt (r=−0.5, p<0.01). There were no complications reported and there were no warnings issued by the dialyser and it was not necessary to make changes to the Ultracontrol® system. As coagulation problems did not occur the dose of heparin was not changed.
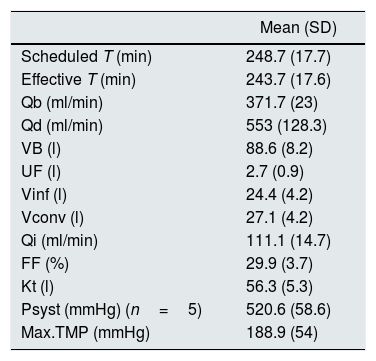
Descriptive results of the dialysis sessions (n=264 sessions).
| Mean (SD) | |
|---|---|
| Scheduled T (min) | 248.7 (17.7) |
| Effective T (min) | 243.7 (17.6) |
| Qb (ml/min) | 371.7 (23) |
| Qd (ml/min) | 553 (128.3) |
| VB (l) | 88.6 (8.2) |
| UF (l) | 2.7 (0.9) |
| Vinf (l) | 24.4 (4.2) |
| Vconv (l) | 27.1 (4.2) |
| Qi (ml/min) | 111.1 (14.7) |
| FF (%) | 29.9 (3.7) |
| Kt (l) | 56.3 (5.3) |
| Psyst (mmHg) (n=5) | 520.6 (58.6) |
| Max.TMP (mmHg) | 188.9 (54) |
FF: filtration fraction; Max.TMP: maximum transmembrane pressure; Psyst: system pressure in AK-200; Qb: blood flow; Qd: dialysis flow; Qi: mean infusion rate; SD: standard deviation; T: time; UF: ultrafiltration; VB: blood volume; Vconv: convective volume; Vinf: infusion volume.
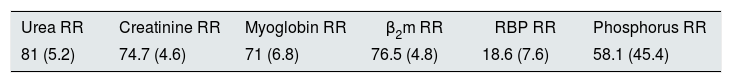
Descriptive results of molecule clearance (%) (n=22 sessions).
| Urea RR | Creatinine RR | Myoglobin RR | β2m RR | RBP RR | Phosphorus RR |
|---|---|---|---|---|---|
| 81 (5.2) | 74.7 (4.6) | 71 (6.8) | 76.5 (4.8) | 18.6 (7.6) | 58.1 (45.4) |
β2m: β2-microglobulin; RBP: retinol-binding protein; RR: reduction ratio.
The results are expressed as a mean (standard deviation).
Finally, Table 4 lists the acute biocompatibility data while Table 5 shows details on inflammation parameters. Both tables show that there is no complement activation when using a cellulose membrane, and there is not worsening in inflammatory molecules after one month of treatment. There was no significant correlation between CRP and the other inflammatory parameters studied.
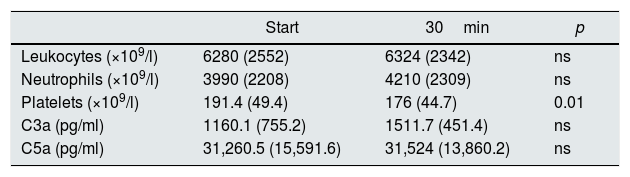
Results on acute biocompatibility with blood samples obtained at the start and after 30min of dialysis (n=22 sessions).
| Start | 30min | p | |
|---|---|---|---|
| Leukocytes (×109/l) | 6280 (2552) | 6324 (2342) | ns |
| Neutrophils (×109/l) | 3990 (2208) | 4210 (2309) | ns |
| Platelets (×109/l) | 191.4 (49.4) | 176 (44.7) | 0.01 |
| C3a (pg/ml) | 1160.1 (755.2) | 1511.7 (451.4) | ns |
| C5a (pg/ml) | 31,260.5 (15,591.6) | 31,524 (13,860.2) | ns |
ns: not significant.
The results are expressed as a mean (standard deviation).
Results on monocytes and inflammatory molecules after one month of use (samples obtained before the first session with Solacea® and at the start of the last session) (n=21 patients).
| Classical (%) | Non-classical (%) | IL-β1 (pg/ml) | IL-6 (pg/ml) | |
|---|---|---|---|---|
| Start | 81.3 (6.8) | 18.6 (6.8) | 0.4 (0.2) | 12.2 (22.4) |
| End | 83.5 (8) | 16.4 (8) | 0.5 (0.1) | 4 (2.2) |
| p | ns | ns | ns | ns |
IL-6: interleukin-6; IL-1β: interleukin-1 beta; ns: not significant.
Our results show that the membrane studied has the necessary characteristics to perform OLHDF; it obtains a suitable Vconv, Kt and RR for different substances, it does not cause technical problems and nursing work is not affected beyond daily clinical practice. Also, the membrane is biocompatible (it does not activate the complement in the short-term or induce changes in the inflammatory profile in the medium term, based on the data obtained on activation of IL and monocytes).
The two clinical parameters used on a day-to-day basis to assess OLHDF's suitability are Kt and Vconv.9,10 The mean Kt reached was 56l, this value is greater than the target suggested in the guidelines, and, although the optimal Vconv remains to be decided, a recent study11 showed that increasing Vconv improves survival linearly between ∼55 and 75l/week; our results indicate that the maximum volume was achieved. The clearance capacity obtained as assessed by RRs for substances of different sizes were also similar to those presented in other studies which feature high-permeability dialysers to perform OLHDF,12,13 taking into account the differences which may be found depending on the dialysis time and the surface used.
According to the EUDIAL group, the dialyser used to perform OLHDF must have the following features: high flux, ultrafiltration coefficient greater than 20ml/mmHg/h/m2, permeability coefficient for β2-microglobulin greater than 0.6, and obtain an effective convective transport greater than 20% of the total processed blood.7 Given that each monitor uses different methods to optimise the FF,14 the results obtained were different depending on which monitor we used, but the results were greater than 27% in all monitors. The classic description of cellulose membranes is that they are thinner than synthetic membranes and have a capillary wall that is uniform and symmetric in composition, without having the necessary resistance to support the high pressures generated in OLHDF.15 However, the ATA® membrane is asymmetrical, which allows to have a low TMP and, therefore, to perform OLHDF achieving adequate clearance, in accordance with the measurement parameters used.
Regarding clinical aspects, there were no significant problems in the daily practice, we observed a well adaptation to the different systems which the monitors use to achieve an adequate Vconv, as reflected in the different FFs obtained. This is important fact, since the absence of alarm warnings improves the nurse's work load and means that the dialyser adapts well to the technique.
The idea persists that the contact of these cellulose membranes with blood causes a major inflammatory response, classifying them as non-biocompatible membranes. This inflammatory response could be acute, with complement activation and leukopaenia after 30min of the session, or have consequences in the longer term through activation of monocytes and cytokines. The severity of this response is due to the hydroxyl groups (OH) within the cellulose molecule. The replacement of these groups with acetate has resulted in modified cellulose triacetate membranes which reduce these responses and make them similar to synthetic dialysers. To determine the membrane's acute biocompatibility, complement activation and leukocytes were measured at 30min, and we found reductions similar to the published with synthetic dialysers. This led us to consider this membrane to be biocompatible and the concept that assumes that cellulose as bioincompatible was dismissed.15
There is a great heterogeneity of circulating monocytes in humans. Circulating monocytes which can be summarised as classical and non-classical according to whether they express greater or lower levels of CD14 and CD16 markers. Classical monocytes are most of the monocytes seen in healthy individuals. Non-classical monocyte populations tend are observed in certain pathological situations, such as cardiovascular disease16 or inflammatory processes.17 Several studies show a reduction in the percentage of non-classical monocytes and of markers of endothelial damage with OLHDF compared to conventional high-flux haemodialysis.18 Elevated levels of proinflammatory cytokines have been associated with greater mortality. Our results reveal that these membranes do not induce changes in these inflammatory markers after one month of treatment, as we have not found significant changes in the percentage of non-classical monocytes or in ILs levels. We therefore believe that this substituted cellulose membrane does not induce inflammatory changes different from those of normal synthetic membranes.
Our study has some limitations, although the number of patients is small, but we believe that the number of sessions performed is high and, therefore, the outcome is representative. The objective was not to compare this membrane with other membranes, but instead to describe the membrane it self. Therefore, the design is suitable. Although the number of sessions performed with some monitors, for example the Nikkiso monitor, was lower, we believe that the results are quite conclusive in terms of good adaptation of the filter to all systems. It is yet to be established if there is loss of albumin, which means that there is a question to be answered, however, we do not have the necessary resources to analyse this. We have found one report in which the loss of Albumin is quantified, and it is less than 2g per session.19
ConclusionsThe new ATA® membrane obtains adequate Kt and Vconv values, without technical problems. We did not find the classic problems attributed to this type of membrane in the biocompatibility parameters studied, making it possible to perform OLHDF, and finding it to be most useful in patients who are allergic to synthetic membranes.
FundingThe study was funded by Nipro, with a financial contribution of €5000. This was allocated entirely to laboratory expenditure. None of the investigators received payment for the study.
Conflicts of interestThe authors declare potential conflicts of interest.
Dr P. de Sequera, Dr M. Albalate and Dr R. Pérez García have participated as speakers at meetings organised by Gambro and Fresenius, and Dr G. Barril has participated at meetings organised by Nikkiso. They charged fees for these talks.
We would like to thank the nursing staff of the Dialysis Unit at Hospital Infanta Leonor, Hospital Príncipe de Asturias and Hospital La Princesa for their collaboration in the study.
Please cite this article as: Albalate Ramón M, Martínez Miguel P, Bohorquez L, de Sequera P, Bouarich H, Pérez-García R, et al. El triacetato de celulosa asimétrico es una alternativa segura y eficaz para la hemodiafiltración en línea. Nefrologia. 2018;38:315–320.