The interest in the preservation of residual kidney function on starting renal replacement therapy (RRT) is very common in techniques such as peritoneal dialysis but less so in haemodialysis (HD). In our center the pattern of incremental dialysis (2HD/week) has been an option for a group of patients. Here we share our experience with this regimen from March 2008.
Material and methodsWe included incident patients with residual diuresis >1000ml/24h, clinical stability, absence of edema, absence of hyperkalaemia >6.5mEq/l and phosphoremia >6mg/dl, with acceptable comprehension of dietetic care. Exclusion criteria were: clinical instability, no dietary or medical compliance and the afore mentioned laboratory abnormalities.
ResultsA total of 24 patients were included in incremental technique. The mean age at start of RRT was 60±15 years. The average time on incremental technique was 19±18 months (range: 7–80), with a mean time on dialysis of 31±23 months (range: 12–86). The reasons for transfer to thrice-weekly HD were: in 6 patients due to laboratory tests, in 2 patients for heart failure events, one for poor compliance and 3 for receiving a kidney graft. The residual diuresis decreased in the first year from 2106±606ml/day to 1545±558 (p=.17) with the urea clearance and calculated residual renal function, basal 5.7±1.5 vs 3.8±1.9ml/min per year (p=.01) and basal 8.9±2.4 vs 6.9±4.3 per year (p=.28), respectively.
ConclusionsIncremental HD treatment, with twice-weekly HD, may be an alternative in selected patients. This approach can largely preserve residual renal function at least for the first year. Although this pattern probably is not applicable to all patients starting RRT, it can and should be an initial alternative to consider.
El interés por preservar la función renal residual una vez iniciado un tratamiento renal sustitutivo (TRS) es notorio en técnicas como la diálisis peritoneal pero es menor en hemodiálisis (HD). En nuestro centro la pauta de diálisis incremental (2HD/semana) ha sido una opción posible para un grupo de pacientes. Mostramos nuestra experiencia con dicha pauta desde marzo de 2008.
Material y métodosIncluimos a pacientes incidentes con diuresis residual >1.000ml/24h, estabilidad clínica, ausencia de edemas, ausencia de hiperpotasemia >6,5mEq/l y de fosforemia >6mg/dl, con aceptable comprensión de los cuidados dietéticos. Fueron criterios de exclusión: la inestabilidad clínica, el no cumplimiento dietético ni médico y las alteraciones analíticas referidas.
ResultadosVeinticuatro pacientes han sido incluidos en la técnica incremental. La edad media al inicio de TRS fue de 60±15 años. El tiempo medio en técnica incremental fue de 19±18 meses (rango: 7-80), con una permanencia media en TRS de 31±23 meses (rango: 12-86). Los motivos de cambio a 3HD/semana fueron: 6pacientes por parámetros analíticos, 2por episodios de insuficiencia cardiaca, uno por mal cumplimiento terapéutico y 3por recibir un injerto renal. La diuresis residual desciende en el primer año de 2.106±606ml/día a 1.545±558 (p=0,07) junto con el aclaramiento de urea y la función renal residual calculada, basal de 5,7±1,5 vs. 3,8±1,9ml/min al año (p=0,01) y basal de 8,9±2,4vs. 6,9±4,3 al año (p=0,28), respectivamente.
ConclusionesLa HD incremental, con 2 sesiones de HD/semana, puede ser una alternativa en un grupo seleccionado de pacientes. Esta modalidad puede preservar la función renal residual en buena medida, al menos durante el primer año. Aunque probablemente no sea aplicable a todos los pacientes que inician TRS, puede y debe ser una alternativa inicial que considerar.
Residual renal function (RRF) is associated with increased survival inpatients with advanced renal disease.1 Numerous studies have shown that it is important to maintain RRF in peritoneal dialysis.2,3 Some studies in haemodialysis (HD) have highlighted the benefits of RRF, however its role has received only limited attention.1,4 Recent studies, have shown improved survival of patients with preserved RRF, and have also enphasized the difficulty involved in the management of RRF in routine clinical practice.5–8
Preserving RRF is associated with better survival and also results in a better tolerance of haemodialysis, improved control of calcium–phosphorus metabolism, better nutritional status, as well as a beneficial effect on anemia and perceived quality of life in patients receiving renal replacement therapy (RRT).9–11
The loss of RRF during the first few months of RRT is well described. This loss of RRF is associated with several factors: episodes of hypotension, volume depletion, activation of inflammatory mediators, biocompatibility of dialyzers and dialysis fluid, and the use of nephrotoxic treatments and contrast media.12,13 Incremental dialysis, starting with twice-weekly sessions and gradually adjusting the length of dialysis under close medical supervision, is well tolerated and the residual urine output is preserved without complications.1,6,7,14
In our center, we decided to introduce incremental HD at the commencement of RRT. To do so we followed the kinetic model developed by Gotch in 1985, in which adequate dialysis is achieved provided if urea clearance does not fall below 2.5ml/min.15,16 In the present study, we describe how we have implemented this strategy in our hospital over the past 7 years. Our objective is to analyze the evolution of patients with incremental HD particularly their residual urine output, the clinical features and analytical parameters.
Material and methodsThis is a retrospective study of a number of case series without a control group. All patients starting incremental RRT since 2008 were considered for inclusion. Patients already on dialysis transferred from other centers, and patients who were subsequently included in the peritoneal dialysis program were analyzed.
Inclusion criteria were: 24h residual urine output of at least 1000ml, clinically stable, no evidence of volume overload or edema. Exclusion criteria were: unstable clinical status, 24h diuresis of less than 1000ml or the presence of laboratory abnormalities such as serum phosphate concentration persistently above 6mg/dl or serum potassium level above 6.5mEq/l. Other exclusion criteria were: non-compliance or being unable to follow instructions and nephrotic proteinuria at the start of RRT. Data from patients who received incremental HD for less than 6 months were not included in the final analysis.
In our center, the criteria for starting RRT with HD are: asymptomatic patient with estimated glomerular filtration rate (GFR) of <6ml/min, calculated using the MDRD or patients with higher GFR with symptoms that do not respond to medical treatment, uremic symptoms or poor control of analytical parameters. Patients starting RRT on an outpatient basis begin on twice-weekly incremental dialysis sessions (Monday and Friday or Tuesday and Saturday) lasting at least 180min por session if the weight is less than 60kg and 210min if they are above 60kg. Following 2006 KDOQI guidelines, after 2–3 weeks the RRF is reassessed together with the determination of residual urine output, conventional analytical parameters along and a physical exam.17 Patients starting RRT due to hospitalization follow the regime recommended by the nephrologist based on their clinical situation. Once the clinical situation has been stable for 2–3 weeks, they are re-evaluated following the same protocol applied to non-hospitalised patients. Provided they meet the inclusion criteria, they are changed to twice-weekly HD with corresponding follow-up.
To considered a patient from our outpatient advanced chronic kidney disease (ACKD) the patient had to be followed for at least 3 months independently of whether the patient had a permanent vascular access.
Once RRT has started, residual urine output was re-evaluated every 2 months, using the 24h urine output from the day preceding the first weekly dialysis. In addition, analytical parameters were monitored every month, the dialysis dose was calculated bi-monthly using the simplified single-pool Kt/V Daugirdas formula, the urea reduction ratio (URR) was calculated every two months, and dialysis adequacy was monitored daily using the standardized formula for urea clearance (Kt and Kt/V). Twice-weekly HD was maintained provided that residual urine output was greater than 1000ml/24h, urea clearance above 2.5ml/min, no evidence of edema or volume overload, and no analytical parameters are persistently outside the recommended range. Blood pressure was measured at the beginning of the dialysis session.
To prevent hypotension the maximum ultrafiltration (UF) rate was set at 1500–2000cc per session, and it was re-evaluated on the basis of edema or volume overload. The “dry” weight was evaluated every week based on physical examination and estimated UF. All patients were advised on fluid intake and dietary salt and potassium intake. As a rule, furosemide was not started at the commencement of incremental HD but it was continued in patients previously receiving furosemide. All patients were dialysed with ultrapure dialysis fluid and high-flux biocompatible membranes. They initially received dialysate with calcium 2.5mEq/l and potassium 1.5mEq/l, at a temperature of 35.5°C, if well tolerated.
The analysis of baseline, 6 month and 1 year parameters included data from patients who started on twice-weekly dialysis, irrespective of whether they were eventually changed to thrice-weekly HD within the year.
Statistical analysisResults are shown as mean±standard deviation, or as median and range when required. All the data analyzed were normally distributed, so parametric tests were used. Means were compared using the Student's t-test for paired data. A p value <0.05 was considered statistically significant.
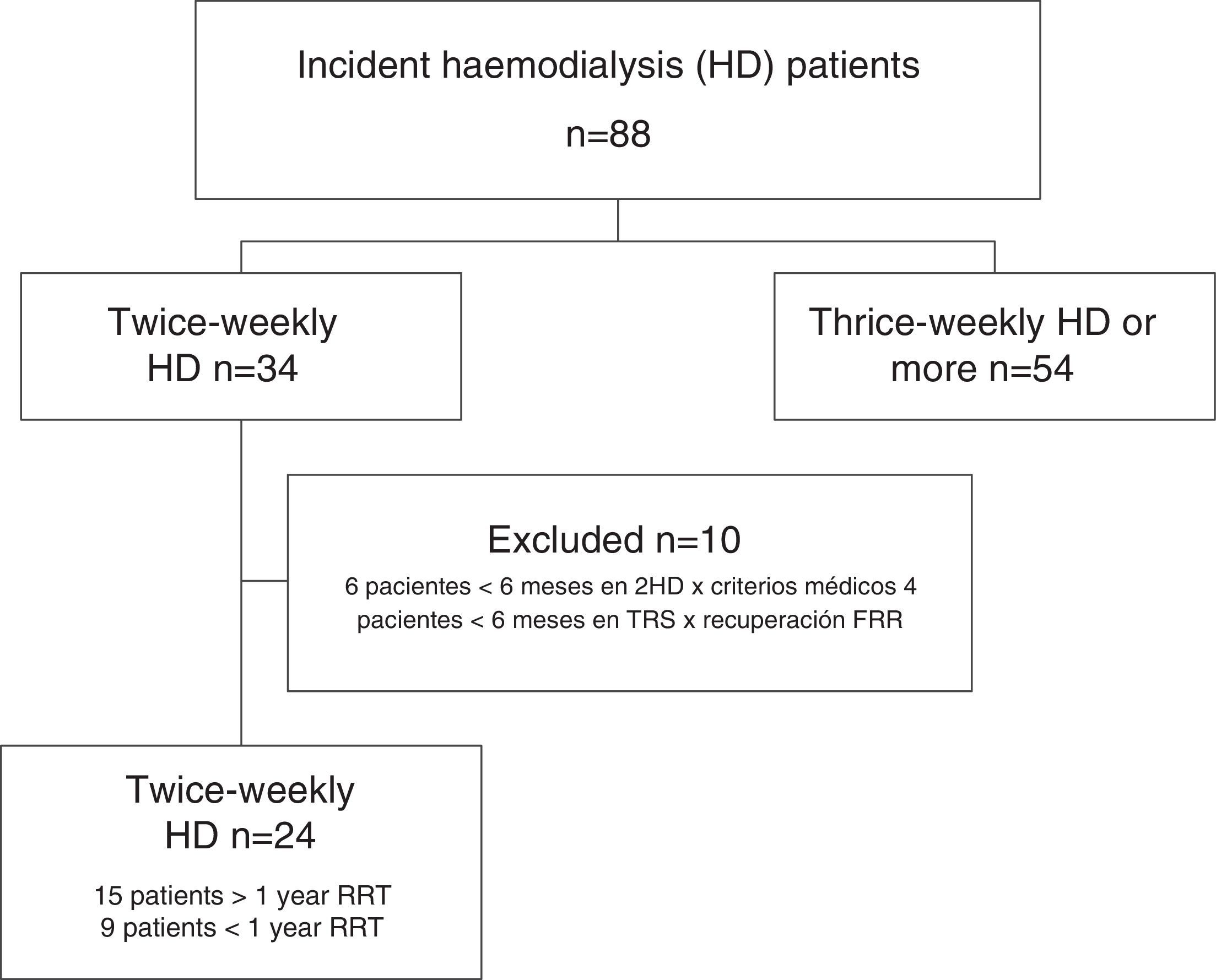
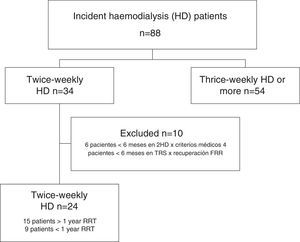
ResultsData from all incident HD patients treated since the inauguration of the Haemodialysis Unit in March 2008 were reviewed. As of September 2015, a total of 34 patients had started incremental twice-weekly HD. Ten patients were excluded because did not remain on incremental HD for at least 6 months. Six patients remained on incremental HD for less than 6 months due to volume overload, poorly controlled clinical parameters, or excessive weight gain. In 4 patients, there was an increase in RRF after starting incremental HD and allowed a temporal discontinuation of RRT was temporarily discontinued (at the end of the study, 3 of these patients had CKD stage 4–5 with no need for RRT, and the fourth patient remained dialysis-free for 18 months, but he had to resumed thrice-weekly HD after a myocardial infarction).
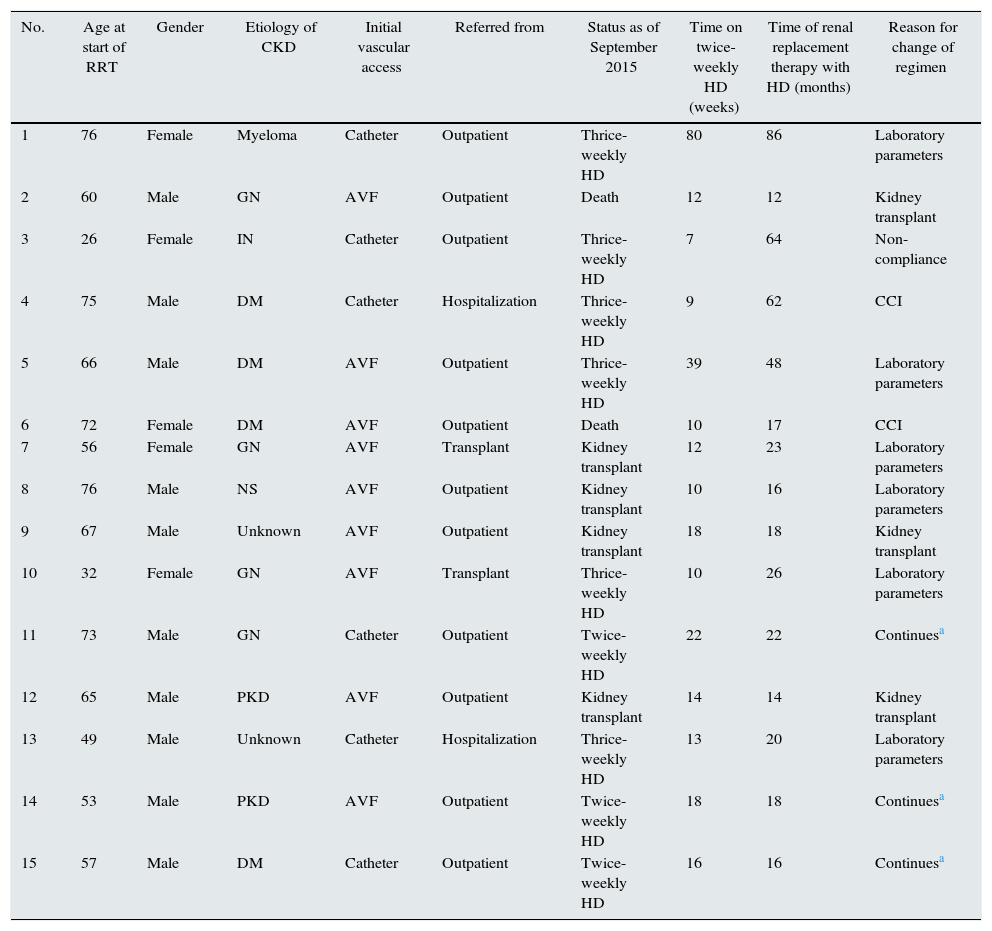
Patients analyzed were those that remained on incremental HD for at least 6 months and on RRT for at least 12 months by the end of the data collection period (1 September 2015). Ten patients had been on incremental HD for at least 1 year, and another 5 had completed more than 6 months (Fig. 1). Of the 24 patients enrolled, 9 receiving twice-weekly HD were ultimately excluded from the analysis because they received less than 12 months by the end of study. Finally, data from 15 patients who had received at least 12 months of RRT, 10 men and 5 women with a mean age at onset of RRT of 60 (15 years), were included in the analysis. Table 1 shows patient demographics and origin of kidney disease.
Characteristics and evolution of patients receiving incremental HD.
| No. | Age at start of RRT | Gender | Etiology of CKD | Initial vascular access | Referred from | Status as of September 2015 | Time on twice-weekly HD (weeks) | Time of renal replacement therapy with HD (months) | Reason for change of regimen |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 76 | Female | Myeloma | Catheter | Outpatient | Thrice-weekly HD | 80 | 86 | Laboratory parameters |
| 2 | 60 | Male | GN | AVF | Outpatient | Death | 12 | 12 | Kidney transplant |
| 3 | 26 | Female | IN | Catheter | Outpatient | Thrice-weekly HD | 7 | 64 | Non-compliance |
| 4 | 75 | Male | DM | Catheter | Hospitalization | Thrice-weekly HD | 9 | 62 | CCI |
| 5 | 66 | Male | DM | AVF | Outpatient | Thrice-weekly HD | 39 | 48 | Laboratory parameters |
| 6 | 72 | Female | DM | AVF | Outpatient | Death | 10 | 17 | CCI |
| 7 | 56 | Female | GN | AVF | Transplant | Kidney transplant | 12 | 23 | Laboratory parameters |
| 8 | 76 | Male | NS | AVF | Outpatient | Kidney transplant | 10 | 16 | Laboratory parameters |
| 9 | 67 | Male | Unknown | AVF | Outpatient | Kidney transplant | 18 | 18 | Kidney transplant |
| 10 | 32 | Female | GN | AVF | Transplant | Thrice-weekly HD | 10 | 26 | Laboratory parameters |
| 11 | 73 | Male | GN | Catheter | Outpatient | Twice-weekly HD | 22 | 22 | Continuesa |
| 12 | 65 | Male | PKD | AVF | Outpatient | Kidney transplant | 14 | 14 | Kidney transplant |
| 13 | 49 | Male | Unknown | Catheter | Hospitalization | Thrice-weekly HD | 13 | 20 | Laboratory parameters |
| 14 | 53 | Male | PKD | AVF | Outpatient | Twice-weekly HD | 18 | 18 | Continuesa |
| 15 | 57 | Male | DM | Catheter | Outpatient | Twice-weekly HD | 16 | 16 | Continuesa |
AVF: arteriovenous fistula; CHF: congestive heart failure; CKD: chronic kidney disease; DM: diabetes mellitus; GN: glomerulonephritis; HD: haemodialysis; IN: interstitial nephritis; NS: nephrosclerosis; PKD: polycystic kidney disease; RRT: renal replacement therapy; VA: vascular access.
Two patients started RRT without previous follow-up as an outpatient, and were placed on twice-weekly HD after admission. Nine patients began RRT with an arteriovenous fistula (AVF), while the other 6 patients needed a tunneled catheter.
Mean time on incremental HD was 19 months (18 months) (median: 13; range: 7–80), and patients remained on RRT for 31 months (23 months) (median: 20; range: 12–86).
Two patients came from the kidney transplant unit with chronic graft dysfunction. In these patients, decreased residual urine output and worsening of analytical parameters required conversion to thrice-weekly HD at 10 and 12 months respectively.
The reasons for changing HD regimen are shown in Table 1. Six patients required conversion to thrice-weekly HD due to abnormal analytical parameters and 2 due to episodes of heart failure (one due to myocardial ischemia and another due to a respiratory infection) that required more strict UF and had a concomitant loss of residual urine output. In 1 case, non-compliance with the pharmacological therapy leading to persistently high levels of phosphorus and potassium called for conversion to thrice-weekly HD. In 3 cases, incremental HD was discontinued due to kidney transplant after 12, 18 and 14 months on this regime.
Two patients died during follow-up, both after discontinuation of incremental HD; one of them due to infection several months after receiving a kidney transplant, and in the other one the cause of death brain hemorrhage that occurred after 7 months of thrice-weekly and a total of 17 months on RRT.
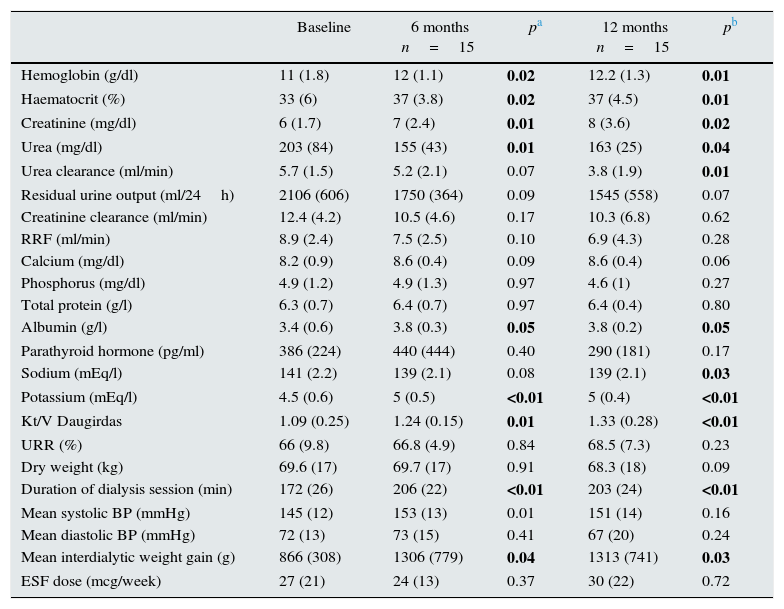
Analytical data and dialysis parameters are shown in Table 2. Average initial residual urine output was 2106±606ml/day that gradually declined over the first year but the average urine output remained over 1l at 12 months, 1545±558ml/day (p=0.07). This observation was consistent with the parallel decrease in urea clearance, from 5.7±1.5 vs to 3.8±1.9ml/min at 12 months (p=0.01). The reduction in reduction was also related to a loss of RRF (ml/min), from 8.9±2.4 at baseline to 6.9±4.3 after 1 year (p=0.28). We also observed that this occurred in parallel with the increased duration of each HD session, 172±26min vs 203±24min at 1 year of follow-up (p=0.001); together with an increased in the dose of dialysis based on Kt/V Daugirdas and URR; the respective calculated dose of dialysis were, 1.09±0.25 and 66±9.8 at baseline vs 1.33±0.28 and 68.5±7.3 at 12 months, (p=0.03 and p=0.23, respectively).
Dialysis and analytical parameters at baseline, 6 months and 1 year of renal replacement therapy.
| Baseline | 6 months n=15 | pa | 12 months n=15 | pb | |
|---|---|---|---|---|---|
| Hemoglobin (g/dl) | 11 (1.8) | 12 (1.1) | 0.02 | 12.2 (1.3) | 0.01 |
| Haematocrit (%) | 33 (6) | 37 (3.8) | 0.02 | 37 (4.5) | 0.01 |
| Creatinine (mg/dl) | 6 (1.7) | 7 (2.4) | 0.01 | 8 (3.6) | 0.02 |
| Urea (mg/dl) | 203 (84) | 155 (43) | 0.01 | 163 (25) | 0.04 |
| Urea clearance (ml/min) | 5.7 (1.5) | 5.2 (2.1) | 0.07 | 3.8 (1.9) | 0.01 |
| Residual urine output (ml/24h) | 2106 (606) | 1750 (364) | 0.09 | 1545 (558) | 0.07 |
| Creatinine clearance (ml/min) | 12.4 (4.2) | 10.5 (4.6) | 0.17 | 10.3 (6.8) | 0.62 |
| RRF (ml/min) | 8.9 (2.4) | 7.5 (2.5) | 0.10 | 6.9 (4.3) | 0.28 |
| Calcium (mg/dl) | 8.2 (0.9) | 8.6 (0.4) | 0.09 | 8.6 (0.4) | 0.06 |
| Phosphorus (mg/dl) | 4.9 (1.2) | 4.9 (1.3) | 0.97 | 4.6 (1) | 0.27 |
| Total protein (g/l) | 6.3 (0.7) | 6.4 (0.7) | 0.97 | 6.4 (0.4) | 0.80 |
| Albumin (g/l) | 3.4 (0.6) | 3.8 (0.3) | 0.05 | 3.8 (0.2) | 0.05 |
| Parathyroid hormone (pg/ml) | 386 (224) | 440 (444) | 0.40 | 290 (181) | 0.17 |
| Sodium (mEq/l) | 141 (2.2) | 139 (2.1) | 0.08 | 139 (2.1) | 0.03 |
| Potassium (mEq/l) | 4.5 (0.6) | 5 (0.5) | <0.01 | 5 (0.4) | <0.01 |
| Kt/V Daugirdas | 1.09 (0.25) | 1.24 (0.15) | 0.01 | 1.33 (0.28) | <0.01 |
| URR (%) | 66 (9.8) | 66.8 (4.9) | 0.84 | 68.5 (7.3) | 0.23 |
| Dry weight (kg) | 69.6 (17) | 69.7 (17) | 0.91 | 68.3 (18) | 0.09 |
| Duration of dialysis session (min) | 172 (26) | 206 (22) | <0.01 | 203 (24) | <0.01 |
| Mean systolic BP (mmHg) | 145 (12) | 153 (13) | 0.01 | 151 (14) | 0.16 |
| Mean diastolic BP (mmHg) | 72 (13) | 73 (15) | 0.41 | 67 (20) | 0.24 |
| Mean interdialytic weight gain (g) | 866 (308) | 1306 (779) | 0.04 | 1313 (741) | 0.03 |
| ESF dose (mcg/week) | 27 (21) | 24 (13) | 0.37 | 30 (22) | 0.72 |
BP: blood pressure; ESF: erythropoiesis stimulating factors; URR: urea reduction ratio; RRF: calculated residual renal function (CrCl+UreaC)/2.
At the beginning of the study, 7 patients were receiving loop diuretics (80–40mg furosemide); at 1 year, 5 patients with preserved residual urine output received the diuretic.
Average baseline weight (kg) at pre- dialysis session was 69.6 (17), no significant change was observed after 12 months 68.3±18kg (p=0.096). Average interdialyctic weight gain (kg) increased significantly during the first year from was 866±308 to 1313±741 (p=0.033). Serum total protein levels did not change, although serum albumin concentration increased significantly at 12 months.
Blood pressure was similar at baseline as compared with 12 months; average baseline systolic blood pressure (mmHg) was 145±12mmHg vs 151±14 after 12 months (p=0.165) and the average baseline diastolic at baseline and one year were 72±13 vs and 67±20 respectively (p=0.243).
The hemoglobin and haematocrit improved after 1 year, with no significant increase in mean dose of erythropoiesis stimulating factors (ESF), that in our case was darbepoetin (mcg/week), 27±21 and 30±22mcg/semana; p=0.725.
The control of seric phosphate and PTH levels improved after 1 year, but the differences were not statistically significant.
DiscussionThrice-weekly hemodialysis sessions are generally recommended by the guidelines. In some countries, incremental twice-weekly HD regimens are considered suboptimal, while in others, with limited resources, is the only option.5,18,19 Preserving residual urine output is an important goal in peritoneal dialysis, however this concern is not common in haemodialysis.2,3,20 Both Vilar et al. and Fernández-Lucas et al. showed that patients starting on twice-weekly incremental HD had better survival and higher residual urine output than patients starting with a thrice-weekly regimen.1,7 Our study does not allow conclusion of patients survival. However, even with the limitation of the present work: small sample size, short follow-up, patient selection bias and absence of control group, we found that twice weekly HD helps to maintain acceptable residual kidney function for one year at least which is beneficial for patients in RRT patients.
In HD patients significant RRF is associated with lower mortality irrespective of other conventional risk factors.4,21 Strategies aimed at preserving RRF, such as incremental dialysis, should be consider as an option at the beginning of RRT. Lin et al., in 2009, found that patients with relatively high urine output on a twice-weekly HD regime maintained adequate dialysis parameters and the RRF is preserved for longer than those on three times a week HD.14 However, there are still questions about the benefit of this strategy, particularly if it carries other potential risks, such as volume overload or infradialysis.22,23 Some authors suggest the need for randomized studies comparing HD regimens, conducting trials that are large enough to support conclusions would be both complex and costly.5
There is no uniformity in how to monitor patient response to incremental HD. We rely on clinical criteria, with particular emphasis on physical examination to detect volume overload and the most widely accepted analytical parameters, such as urea clearance and residual urine output.1,7,24 As shown by Teruel et al.,8 careful application and monitoring of incremental HD may preserve RRF as efficiently as peritoneal dialysis. In this 2013 study, the authors reported that preservation of RRF in patients undergoing incremental HD was comparable to that of patients receiving peritoneal dialysis. Recently, Kalantar-Zadeh et al. proposed a set of clinical and laboratory criteria for twice-weekly HD patients, based mainly on urine output.5 Although control of urine output is important, other parameters should also be considered; we, like other authors, monitor the urea clearance.8 We also evaluated other conventional parameters used for dialysis monitoring, and although the ultimate aim is to preserve RRF, the presence of parameters the limit explain the gradual increase the dialysis dose which is associated to a decline in RRF. Some studies have weighed up the benefits of preservation of RRF against conventional dialysis adequacy as a factor to take into account in incremental HD.5,25 We use conventional parameters including residual urine output to ensure dialysis dosage, even at the risk of loss of RRF.26
In our series, unlike others, furosemide is not routinely added to the treatment in patients starting on incremental HD particularly if they were already polymedicated unless if they were already taking this medication.7,27 Although furosemide can improve fluid balance, some authors have shown that it does not improve RRF.28
Other aspects that should be taking into consideration to prevent loss of RRF are: improved biocompatibility of dialysis membranes, the availability of ultrapure dialysis fluids and advances made in the technology used to control dialysis sessions. All these improve dialysis tolerance and prevent factors that are potentially damaging to RRF.13,29,30 In 2002, McKane et al., achieved an identical decline in RRF using high-flux biocompatible dialysis vs peritoneal dialysis.31 In our series, all patients were dialysed using high-flux membranes and ultrapure fluids.
Instability during dialysis and episodes of hypotension can also accelerate the decline of RRF. For this reason, we limit the rate of UF to a maximum per session provided this is tolerated by the patient (no edema or other signs of volume overload). In the absence of bioimpedance measurements, even though dialysis machines are equipped with automatic volume measurement systems, we established that a persistent poorly controlled “dry” weight is a criteria for conversion to thrice-weekly HD. This limitation may have prevented us from achieving the more objective dry weight adjustment achieved by other authors, and also probably prevented us from maximizing the potential of incremental HD.7
Anemia is another parameter to be considered in incremental HD. We achieved better control of hemoglobin levels, albeit using similar doses of ESF, other authors have reported lower dose requirements.7 Similarly, serum phosphate and PTH levels were well controlled but the difference was not statistically significant. There was a mean 1.3kg reduction in “dry” weight over 12 months, although this was not statistically significant. Nevertheless, we did observe a slight, though significant, improvement in serum albumin levels. This could be related with a better nutritional status resulting from preserved RRF, as reported in other series.32
Finally, 5 patients from our series underwent kidney transplantation; 3 were on an incremental HD regimen at the time of transplant, and although the other 2 had been changed to thrice-weekly HD, they had preserved residual urine output. Several studies have showed that residual urine output is associated with better graft survival and less graft-related urological complications, suggesting the preserving RRF could be beneficial in transplant patients. Incremental HD could predict a favorable outcome in kidney transplant patients.33,34
LimitationsRetrospective design and small sample size are the main limitations. Evolution was biased by our selection of patients that met criteria for a favorable outcome, and this prevents us from extrapolating our results to other RRT populations undergoing 3 or more HD sessions per week.
The characteristics of our series also prevented us from evaluating certain aspects, such as β2-microglobulin removal, or from obtaining more accurate weight calculations using methods such as bioimpedance or automated control systems. As this was not possible in our center, the lack of data on the removal of medium-sized molecules has limited the number of analysable parameters. Nevertheless, we believe that the satisfactory evolution of our patients, the generation of a clinical protocol and the experience obtained after 7 years using incremental HD provides valuable insight into this dialysis regimen.
In conclusion, twice-weekly HD can preserve residual urine output, at least over the first year of treatment. RRF may contribute to better survival in kidney patients, and may also be beneficial in other contexts. The conventional criteria for dose of dialysis may not be the best for twice-weekly HD, however they should be used as there is no evidence to support the use of alternative parameters. Twice-weekly HD at the commencement of RRT demands close, careful monitoring, but certainly is a valid option in a select group of patients.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Merino JL, Domínguez P, Bueno B, Amézquita Y, Espejo B, Paraíso V. Aplicación de una pauta de hemodiálisis incremental, basada en la función renal residual, al inicio del tratamiento renal sustitutivo. Nefrologia. 2017;37:39–46.