Valacyclovir, a prodrug of acyclovir with greater oral bioavailability, has been widely used in recent years for the treatment of herpes virus infections. After its hepatic metabolism, it is eliminated through glomerular filtration and active tubular secretion.1 Advanced age and renal failure increase the likelihood of side effects, such as neurotoxicity.2,3 If toxicity is suspected, it is essential and early diagnosis and discontinuation of the medication. In selected cases, hemodialysis could accelerate the clearance of the drug and recovery from the toxic effects of the drug.4
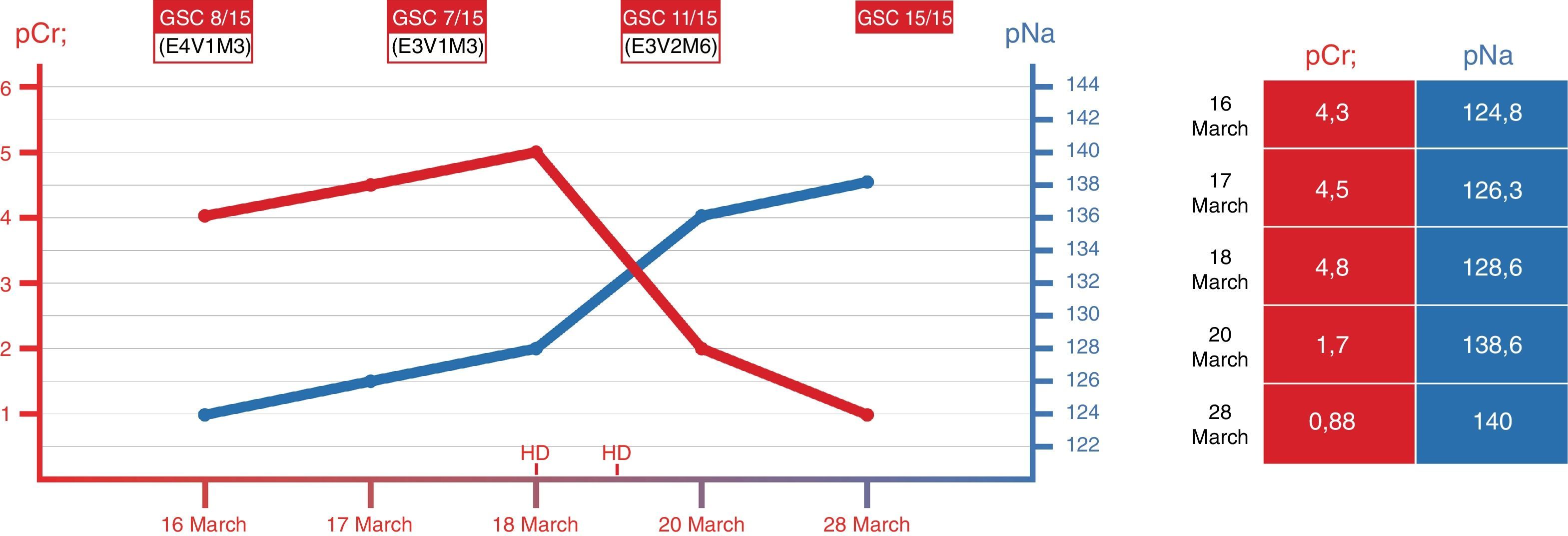
An 87-year-old woman with no relevant past medical history, except hypertension and type 2 diabetes mellitus treated with metformin. She visited his primary care doctor for vesicular lesions along the path of the V1 branch of the left V cranial nerve. The patient was started on oral valacyclovir following the usual regimen (1g/8h). She presented progressive confusional symptoms and abnormal behavior, being transferred to the emergency room on five days after starting the drug. She had no fever, no symptoms of infection and did not present tonic–clonic movements. The physical examination shows no relevant findings except for a Glasgow Coma score of 7/15 (E1V2M4), with no focality and negative meningeal signs. Blood test reveal a moderate hyponatremia, renal failure with mild hyperkalemia without electrocardiographic repercussion, and metabolic acidosis with normal anion GAP. The urine analysis was normal, and the urine ions did not suggest extracellular volume deletion. There was no reduction of diuresis, and kidney ultrasound shows kidneys without signs of chronic renal failure without dilation of the excretory system. Chest radiography and cranial computed tomography without intravenous contrast did not show pathological findings. Lumbar puncture ruled out viral meningoencephalitis.
After the initial administration of 100cc of hypertonic saline (3%), there was no change in the level of consciousness, despite that the serum sodium was practically corrected. Neurotoxicity for valacyclovir was suspected and it was decided to perform a first conventional hemodialysis session of 2.5h that produced a slight improvement in the neurological situation (Glasgow coma scale of 11/15). A second hemodialysis session was performed, this time of 4h, with clear clinical improvement. Within a few days, the patient was discharged with normal renal function and complete neurological recovery (Fig. 1).
Valacyclovir is the L-valine ester of acyclovir, active against the herpes virus. It has oral bioavailability; quickly and almost completely becomes acyclovir after a first-pass mainly hepatic.1 The elimination half-life is approximately 3h, which is increased in the presence of renal failure. Elderly patients seem more vulnerable to the adverse effects of the drug, mainly neurological effects, so it should be used with caution, and requires close monitoring and ensure adequate hydration.
Valacyclovir neurotoxicity was first described in 1998 by Linssen-Schuurmans et al.2 Since then more than 20 cases have been reported. Usually occurs 72h after initiation of the drug, and usually resolves 4 days after the discontinuation of the same, although sometimes it may take up to 2 weeks. The majority of cases occur in patients with renal insufficiency, either chronic (present at the beginning of the drug) or acute, due to tubular precipitation of acyclovir crystals and/or acute tubulointerstitial nephritis. The clinical spectrum ranges from mild symptoms, such as confusion, photophobia or dysarthria, to symptoms of extreme severity, such as hallucinations, seizures and coma. The differential diagnosis of neurotoxicity by valacyclovir should be established with entities such as viral encephalitis, mental disorders or cerebrovascular diseases. In the case of our patient, lumbar puncture and cranial computed tomography were performed, ruling out the infectious etiology, as well as the presence of space-occupying lesions or acute vascular disease. If possible, levels of valacyclovir should be requested (therapeutic range: 2–4mcg/mL). And in cases without clear diagnosis, serum and/or cerebrospinal fluid levels of 9-carboxymethoxymethylguanine can be measures and are elevated in case of toxicity by valacyclovir.5,6 The treatment consist in discontinuation of the drug, with recovery of acute renal failure and neurological alterations in a period that in most cases ranges between 48h and 2 weeks. Treatment with hemodialysis in selected cases has shown a significant reduction in serum levels of the drug, and improvement in symptomatology.3,4 Peritoneal dialysis, however, does not seem to be effective.
In conclusion, valacyclovir is a drug that should be prescribed with caution in the elderly population, since it can produce acute renal failure that delays its elimination, increasing the risk of adverse effects, such as neurotoxicity. Adequate hydration should be ensured and, if possible, monitoring of renal function. With the appearance of neurological symptoms, it is recommended to suspend the drug and rule out other entities that have a similar clinical presentation. In selected cases, treatment with hemodialysis may accelerate recovery.
Please cite this article as: Ferreira M, Vega C, Rivas B, Selgas R. Fracaso renal agudo y neurotoxicidad severa tras sobredosis accidental por valaciclovir en población geriátrica: a propósito de un caso. Nefrología. 2018;38:323–325.