Since 2004, various criteria have been proposed to define and grade acute kidney injury (AKI). Nevertheless, fixed criteria for assessing severe sepsis-related AKI have not yet been established.
ObjectivesTo assess the power of the different methods of AKI classification to predict mortality in a cohort of patients with sepsis.
MethodsA prospective study of patients>18 years with septic shock admitted to the intensive care unit (ICU) of our hospital from April 2008 to September 2010 was conducted. Plasma creatinine levels were measured daily. Patients were classified retrospectively according to RIFLE, AKIN, KDIGO and creatinine kinetics (CK) criteria.
ResultsThe percent of AKI rate according to the different criteria was 74.3% for RIFLE, 81.7% for AKIN, 81.7% for KDIGO and 77.5% for CK. AKI staging by RIFLE (OR 1.452, p=0.003), AKIN (OR 1.349, p=0.028) and KDIGO criteria (OR 1.452, p=0.006), but not CK criteria (OR 1.188, p=0.148) were independently related to in-hospital mortality.
ConclusionsA high rate of patients with severe sepsis developed AKI, which can be classified according to different criteria. Each stage defined by RIFLE, AKIN and KDIGO related to a higher risk of in-hospital mortality. In contrast, the new CK criteria did not relate to higher mortality in patients with severe sepsis and this classification should not be used in these patients without having validated its suitability with further studies.
Desde 2004 se han propuesto diversos criterios para definir y estadiar el fracaso renal agudo (FRA), sin embargo, no se conoce cuál de ellos debe ser empleado cuando se desarrolla FRA en el contexto de la sepsis grave.
ObjetivoValorar la capacidad predictiva de mortalidad en una cohorte de pacientes con sepsis de los distintos métodos de clasificación del FRA.
MétodosEstudio prospectivo de los pacientes>18 años ingresados en la Unidad de Cuidados Intensivos (UCI) de nuestro hospital desde abril de 2008 hasta septiembre de 2010 con shock séptico. La creatinina plasmática se determinó diariamente en UCI. Los pacientes se clasificaron de forma retrospectiva según las clasificaciones RIFLE, AKIN, KDIGO y cinética de la creatinina (CK).
ResultadosEl porcentaje de pacientes que desarrolló FRA según cada clasificación fue: 74,3% RIFLE; 81,7% AKIN; 81,7% KDIGO y 77,5% CK. Cada estadio de FRA por RIFLE (OR 1,452; p=0,003), por AKIN (OR 1,349; p=0,028) y por KDIGO (OR 1,452; p=0,006) se relacionaba de forma independiente con la mortalidad intrahospitalaria, pero no por CK (OR 1,188; p=0,148).
ConclusionesUn porcentaje elevado de pacientes con sepsis grave desarrolla FRA que se puede clasificar según los distintos métodos propuestos. Los estadios de las clasificaciones RIFLE, AKIN y KDIGO se relacionan con un mayor riesgo de muerte intrahospitalaria. Por el contrario, la nueva definición de CK no se relaciona con una mayor mortalidad y no se debería usar en estos pacientes con sepsis grave sin confirmar su utilidad en estudios posteriores.
Acute kidney injury (AKI) is one of the most serious complications in hospitalised patients. AKI patients are at increased risk of death, both short and long term, at increased risk of developing chronic kidney disease, and they consume a substantial amount of healthcare resources.1–9 Close to half of all cases of AKI in critically ill patients are caused by sepsis.10,11 The high mortality rates in sepsis patients are largely due to the development of AKI which occur in 1/3 to 2/3 of cases, depending on the series.12–15
Different classifications have traditionally been used to define and grade AKI. However, it was eventually recognised that standardised definitions of AKI were required so it could be easily applied in routine practice; also, there was a need for epidemiological and research studies to be carried out.16 As a result, since 2004, four systems for defining and staging AKI have been proposed.17–20 The various studies that have compared the validity of these classification modalities to predict outcomes of AKI have not found any one method to offer substantial advantages over the others.21 The classification system based on creatinine kinetics (CK) seems to offer some advantages, especially in patients with previous chronic kidney disease.22,23 However, in patients with sepsis, creatinine synthesis is profoundly diminished24 and patients are often haemodiluted, which would alter the utility of using a classification system based on absolute changes in the creatinine values. The aim of our study was to assess the degree of agreement in the diagnosis and classification of AKI and the predictive power for mortality in a cohort of patients with sepsis between different AKI classification methods.
MethodsWe prospectively included all patients over 18 years admitted to the Intensive Care Unit (ICU) at Marqués de Valdecilla University Hospital from April 2008 to September 2010 with septic shock according to the definitions proposed by the SCCM/ESICM/ACCP/ATS/SIS Consensus Conference, i.e. the presence of severe sepsis with hypotension or signs of persistent tissue hypoperfusion that do not respond to intravenous administration of 20ml/kg of fluids and require infusion of vasoactive drugs.25 Patients with renal impairment on renal replacement therapy and kidney transplant recipients were excluded from the study.
Demographic and analytical variables (leukocytes, lactate, base excess, CRP, procalcitonin), use of vasopressors and APACHE-II and SOFA patient classification scores calculated at the time of admission to ICU were all recorded from the medical records. Plasma creatinine was determined daily during the patient's admission to the ICU. Baseline creatinine was considered as the last available creatinine value obtained 7–365 days before admission.26,27 For the 4% of patients who did not have previous values in that period, baseline creatinine was considered as that calculated for a glomerular filtration rate of 75ml/min/1.73m2 estimated using the MDRD-4 equation, following the ADQI group recommendation.28 Patients were retrospectively classified according to the RIFLE, AKIN, KDIGO and CK classifications according to the definitions previously proposed.20,26
The continuous variables were expressed as mean±standard deviation and the qualitative variables as their absolute values or percentages. Proportions were compared using the chi-squared test. The degree of agreement to classify patients by stages between the different classification methods was estimated using Cohen's weighted kappa. The ability to discriminate for the in-hospital mortality risk for each classification method was measured by the area under the ROC curve (AUC-ROC). Logistic regression analysis was performed to estimate the risk of death in the hospital according to each AKI classification method, unadjusted and adjusted, for APACHE-II and SOFA.
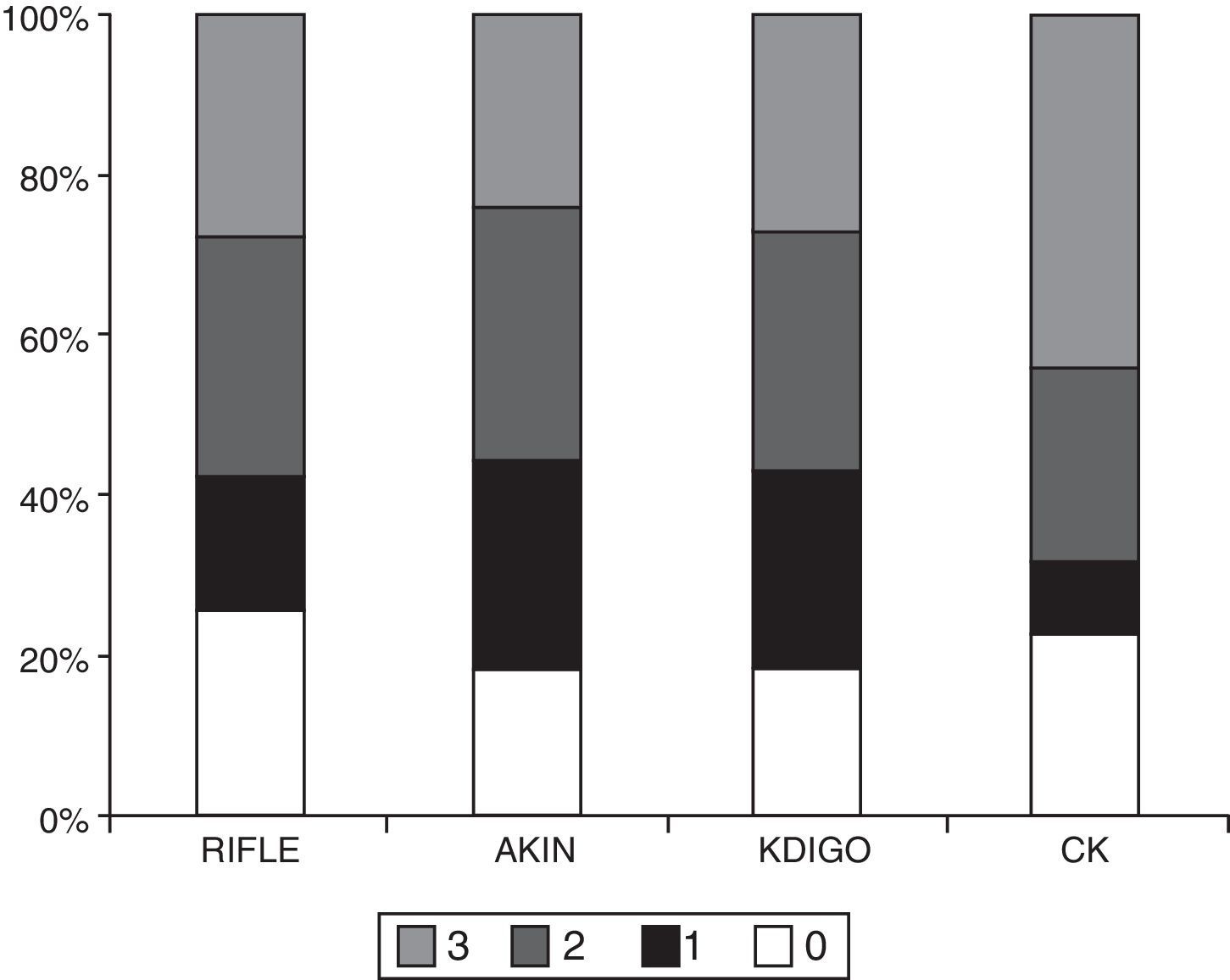
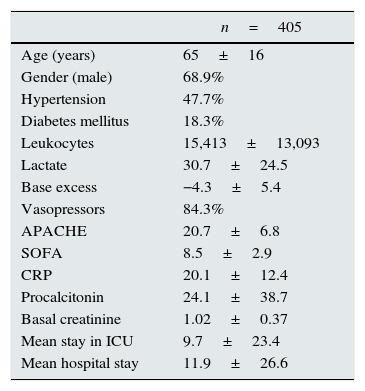
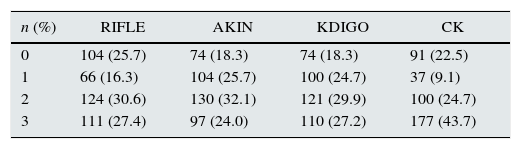
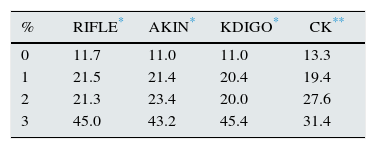
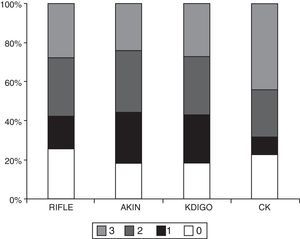
ResultsThe main characteristics of the patients (n=405) are described in Table 1. The percentage of patients who developed AKI according to each classification method was as follows: 74.3% RIFLE; 81.7% AKIN; 81.7% KDIGO and 77.5% CK. The number of patients in each stage according to the different classifications is shown in Table 2 and Fig. 1. The degree of agreement between RIFLE and AKIN was 95.7% (kappa index 0.895; p<0.001), between RIFLE and KDIGO 97.1% (kappa index 0.929; p<0.001), between RIFLE and CK 85.4% (kappa index 0.666; p<0.001), between AKIN and KDIGO 98.3% (kappa index 0.956; p<0.001), between AKIN and CK 85.7% (kappa index 0.664; p<0.001) and between KDIGO and CK 85.5% (kappa index 0.658; p<0.001).
Main patient characteristics.
| n=405 | |
|---|---|
| Age (years) | 65±16 |
| Gender (male) | 68.9% |
| Hypertension | 47.7% |
| Diabetes mellitus | 18.3% |
| Leukocytes | 15,413±13,093 |
| Lactate | 30.7±24.5 |
| Base excess | −4.3±5.4 |
| Vasopressors | 84.3% |
| APACHE | 20.7±6.8 |
| SOFA | 8.5±2.9 |
| CRP | 20.1±12.4 |
| Procalcitonin | 24.1±38.7 |
| Basal creatinine | 1.02±0.37 |
| Mean stay in ICU | 9.7±23.4 |
| Mean hospital stay | 11.9±26.6 |
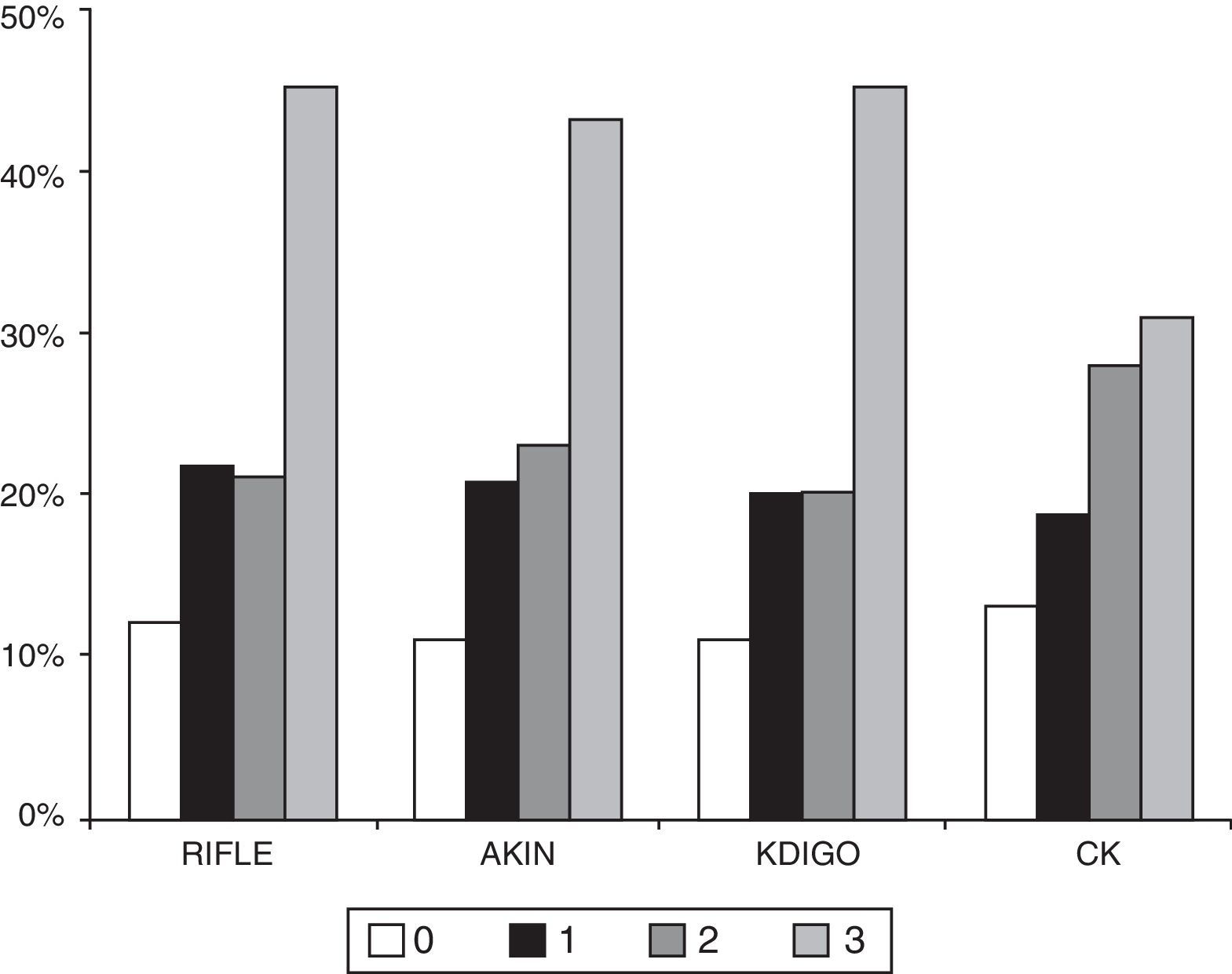
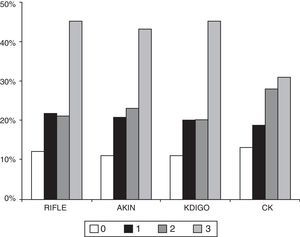
The number of patients who died during hospitalisation was 101 (24.9%) and at three months there were 109 deaths (26.9%). The percentage of patients who died in the hospital was significantly higher in more advance stages of AKI in any of the classification methods (Table 3 and Figure 2). The AUC-ROC for in-hospital mortality for RIFLE was 0.673 (95% CI: 0.612–0.734), AKIN 0.649 (95% CI: 0.588–0.711), KDIGO 0.667 (95% CI: 0.606–0.729), CK 0.600 (95% CI: 0.539–0.661), with no significant differences in the ability to discriminate. Adjusted for the APACHE-II severity rating system, each stage of AKI measured by RIFLE (OR 1.452; 95% CI 1.137–1.854; p=0.003), AKIN (OR 1.349; 95% CI: 1.033–1.761; p=0.028) and KDIGO (OR 1.452; 95% CI: 1.115–1.892; p=0.006) was independently associated with in-hospital mortality. By contrast, although the classification by CK was associated with increased in-hospital mortality in each stage (OR 1.421, 95% CI: 1.151–1.755; p=0.001), the association was not sustained after adjustment by APACHE-II (OR 1.188; 95% CI: 0.941–1.499; p=0.148). The adjustment by SOFA produced similar results.
In the context of severe sepsis, a majority of patients develop AKI. Depending on the definition used, 74–82% of the cohort of ICU patients with sepsis developed AKI. The most sensitive definitions were AKIN and KDIGO while, according to the RIFLE criteria, fewer patients had AKI. The lack of a standardised reference method makes it difficult to estimate the diagnostic accuracy of each classification.16 Most studies comparing RIFLE and AKIN find a higher percentage of patients with AKI if the AKIN criteria are used, both in hospitalised patients in general and patients in ICU or cardiac surgery,16 although this has not been confirmed in all studies.26,27,29 The definition by CK was more sensitive than RIFLE for diagnosing AKI in one study,23 but less sensitive than KDIGO in another.22 Differences in the characteristics of the populations studied, in whether or not to include diuresis in the definition of AKI, in the definition of baseline renal function and in the number of days monitoring creatinine can cause the percentages of patients defined as AKI for each criterion to vary.
Although there are differences in the percentage of diagnoses of AKI, in general, the degree of agreement between the definitions is acceptable. In our study, the degree of agreement was “very high” (kappa>0.8) between RIFLE, AKIN and KDIGO and “good” (kappa 0.6–0.8) between CK and the other classification methods. In previous studies comparing RIFLE and KDIGO, the kappa index was always good or very good; it varied from 0.682 to 0.849.29–32 In the only previous study that analyses the degree of agreement between CK and RIFLE, the values obtained were similar to those reported here (0.67).23 Despite the degree of agreement, it is interesting to note that with the CK classification, most patients with AKI are classified within the maximum degree of severity: 3. Similarly, Liborio et al. found that most patients classified as stage 2 by KDIGO passed to stage 3 using CK22.
It is very common for patients with sepsis to develop AKI and it contributes to an increased risk of death.15 The AKI risk can be reduced, at least partially, by early, goal-directed resuscitation,15 although this last point has not been verified in all studies.33 The increase in mortality is related not only to the diagnosis of AKI but also to its severity.34 Thus, the discrimination ability measured by AUC-ROC was always significant with each of the classifications we analysed and has also been demonstrated in previous studies, with higher values in less heterogeneous clinical settings than sepsis (AUC-ROC for mortality 0.731 for KDIGO and 0.687 for CK after myocardial infarction and 0.852 for RIFLE and 0.887 for CK after cardiac surgery).22,23 Significant differences between the different definitions in their ability to discriminate the risk of death were not found in these studies or in ours.22,23
A recent multicentre, epidemiological study involving 1802 patients in ICUs shows the relationship between the severity of AKI in each of the stages of KDIGO and increased risk of death.34 The quality of the previous studies only provides us with a low-to-moderate level of evidence on the relationship between each RIFLE and AKIN stage and the risk of death.21 In our prospective cohort of patients with sepsis, classifications by RIFLE, AKIN and KDIGO were independently associated with the risk of in-hospital death. The minimal difference in mortality detected between stages 1 and 2 with the three classifications has been previously reported using the AKIN criteria.16,21
In contrast, the stages of severity defined by CK were not independently associated with the risk of death in our population. Only two previous studies have analysed the association between AKI classified by CK and the risk of death. In patients with myocardial infarction, the risk for each stage of CK was significant (OR 5.607, 95% CI: 1.915–16.421, adjusted for APACHE-II) and the findings were similar in a group of patients who had undergone cardiac surgery.22,23 It has been shown in experimental models that sepsis reduces creatinine production and this can lead to kidney damage being underestimated in sepsis more than in other forms of AKI24, especially with a classification that does not include a previous “normal” baseline creatinine value. Similarly, the haemodilution presented by critically ill patients in ICU may vary the effectiveness of each classification. The CK model described by Waikar and Bonventre is based on an “ideal” mathematical simulation that does not take in the whole complexity of severe sepsis.20
In conclusion, a percentage of patients with severe sepsis will develop AKI, which can be diagnosed and classified according to the different proposed methods: RIFLE, AKIN, KDIGO and CK. They all have a high or very high degree of agreement with each other. Using the RIFLE, AKIN and KDIGO classification methods, each stage of greater severity of AKI is associated with an increased risk of in-hospital death, with no significant differences between them. In contrast, the new definition of CK was not associated with increased mortality in our cohort of patients with severe sepsis and should not be used in these patients without being confirmed in future studies.
Conflict of interestsThe authors have no conflicts of interest.
This work has been supported by a grant from the Sociedad Española de Nefrología (S.E.N.) [Spanish Society of Nephrology] 2013–2015.
Please cite this article as: Rodrigo E, Suberviola B, Albines Z, Castellanos A, Heras M, Rodriguez-Borregán JC, et al. Comparación de los sistemas de clasificación del fracaso renal agudo en la sepsis. Nefrologia. 2016;36:530–534.