Switching to cyclosporin A may result in a reversion of tacrolimus-induced diabetes mellitus. However, mechanisms underlying such a reversion are still unknown.
MethodsObese Zucker rats were used as a model for tacrolimus-induced diabetes mellitus. A cohort of 44 obese Zucker rats received tacrolimus for 11 days (0.3mg/kg/day) until diabetes development; then, (a) 22 rats were euthanized at day 12 and were used as a reference group (tacrolimus-day 12), and (b) 22 rats on tacrolimus were shifted to cyclosporin (2.5mg/kg/day) for 5 days (tacrolimus-cyclosporin). An additional cohort of 22 obese Zucker rats received the vehicle for 17 days and was used as a control group. All animals underwent an intraperitoneal glucose tolerance test at the end of the study.
Resultsβ-Cell proliferation, apoptosis and Ins2 gene expression were evaluated. Compared to rats in tacrolimus-day 12 group, those in tacrolimus-cyclosporin group showed a significant improvement in blood glucose levels in all assessment points in intraperitoneal glucose tolerance test. Diabetes decreased from 100% in tacrolimus-day-12 group to 50% in tacrolimus-cyclosporin group. Compared to tacrolimus-day-12 group, rats in tacrolimus-cyclosporin group showed an increased β-cell proliferation, but such an increase was lower than in rats receiving the vehicle. Ins2 gene expressions in rats receiving tacrolimus-cyclosporin and rats receiving the vehicle were comparable.
ConclusionAn early switch from tacrolimus to cyclosporin in tacrolimus-induced diabetes mellitus resulted in an increased β-cell proliferation and reversion of diabetes in 50% of cases.
El cambio a ciclosporina A podría revertir la diabetes inducida por tacrolimus. Sin embargo, los mecanismos de esta reversibilidad se desconocen.
MétodosUsamos como modelo de diabetes inducida por tacrolimus las ratas Zucker obesas. Un grupo de 44 ratas Zucker obesas fue tratado con tacrolimus durante 11días (0,3mg/kg/día) hasta que desarrollaron diabetes; posteriormente, a) 22 fueron sacrificadas a día 12 como grupo referencia (tacrolimus-d12), y b) en otras 22 el tacrolimus fue reemplazado por ciclosporina (2,5mg/kg/día) durante 5días (tacrolimus-ciclosporina). Veintidós ratas Zucker obesas recibieron vehículo durante 17días (grupo control). A todos los animales se les realizó una sobrecarga intraperitoneal de glucosa al final del experimento.
ResultadosSe analizó la proliferación de la célula β, la apoptosis y la expresión del gen Ins2. En el grupo tacrolimus-ciclosporina, los niveles de glucemia mejoraron significativamente en cada punto del test intraperitoneal de glucosa comparados con el grupo tacrolimus-d12. La diabetes se redujo del 100% en los tacrolimus-d12 hasta el 50% en tacrolimus-ciclosporina. La proliferación de las células β en tacrolimus-ciclosporina se incrementó en comparación con tacrolimus-d12, pero fue menor que en los tratados con vehículo. La expresión génica de Ins2 en tacrolimus-ciclosporina fue comparable a los tratados con el vehículo.
ConclusiónEl cambio temprano de tacrolimus por ciclosporina en la diabetes inducida por tacrolimus incrementa la proliferación de la célula β y revierte la diabetes en un 50% de los casos.
Cyclosporine-A (CsA) and tacrolimus (TAC), which are calcineurin inhibitors (CNI), play a major part in immunosuppression after kidney transplantation. TAC is being the most widely used1 as it offers advantages over CsA in terms of better graft function and lower rates of acute rejection,1,2 although it is associated with a higher incidence of post-transplant diabetes mellitus (PTDM).3 The incidence of PTDM ranges between 15 and 25%4–6 and is associated with higher rates of cardiovascular disease and increased healthcare costs.3,7,8 Hence, the study of PTDM induced by TAC is relevant to establish early preventive and therapeutic strategies that reduce these consequences for said population.
According to current guidelines,3,9 PTDM is considered a fait accompli, and its treatment follows the therapeutic rules of type 2 diabetes: oral anti-diabetics and/or insulin.9 However, rarely is action taken upon the compound which most frequently causes PTDM, TAC, that is even though CsA has a lower diabetogenic effect than the former.3,8,9 The change from TAC to CsA for PTDM reversion has rarely been suggested. In a retrospective study of 34 PTDM cases, Ghisdal et al. showed remission rates of 42% one year after the change from TAC to CsA, compared to 0% in the control group, which remained with TAC.10 Nevertheless, to the best of our knowledge, no study has been carried out with animal models to establish the mechanisms due to which a change from TAC to CsA could be a therapeutic alternative in PTDM treatment.
Our group has previously researched the effect of CNI on homeostasis of glucose and on β cells, both in animals with and without insulin resistance.11 In this model, the CNI only induced diabetes in insulin-resistant animals, and TAC induced more diabetes than CsA (100% vs 40%). Additionally, animals with CsA presented less severe alterations in the homeostasis of glucose, according to both intraperitoneal glucose tolerance tests as well as blood sugar levels while fasting. These facts could be explained by a lower reduction of β cell proliferation and of the expression of the insulin gene with CsA than with TAC.11 With said data, the objective of this study is to research whether a change from TAC to CsA in insulin-resistant animals with diabetes induced by TAC could improve the homeostasis of glucose, through the induction of changes to the rates of proliferation and apoptosis of β cell and the expression of the insulin gene.
Material and methodsAnimalsIn a prior study, we researched the effect of CNI on homeostasis of glucose, in animals both resistant and sensitive to insulin, i.e. obese (OZR) and thin Zucker rats (TZR).11 OZR have a homozygous mutation in the leptin receptor (fa/fa),12,13 which results in hyperphagia, obesity, hyperlipidemia, and severe insulin resistance.14,15 OZR, in normal conditions, are able to maintain euglycaemia due to a high proliferation of β cells and due to hypersecretion of insulin.16 It is curious that the CNI only induces diabetes when they are administered to insulin-resistant animals, i.e. OZR, and not to TZR.11 With said data, for this study, we only performed the study on OZR.
We used animals of between four to six weeks of age, with a weight of 200±50g (Charles-River, France). They were confined to adequate cages, at 22°C, with a light-darkness cycle of 12h, and fed with water and food in pellet ad libitum. The ethics committee of the Hospital Universitario de Canarias approved the experiment.
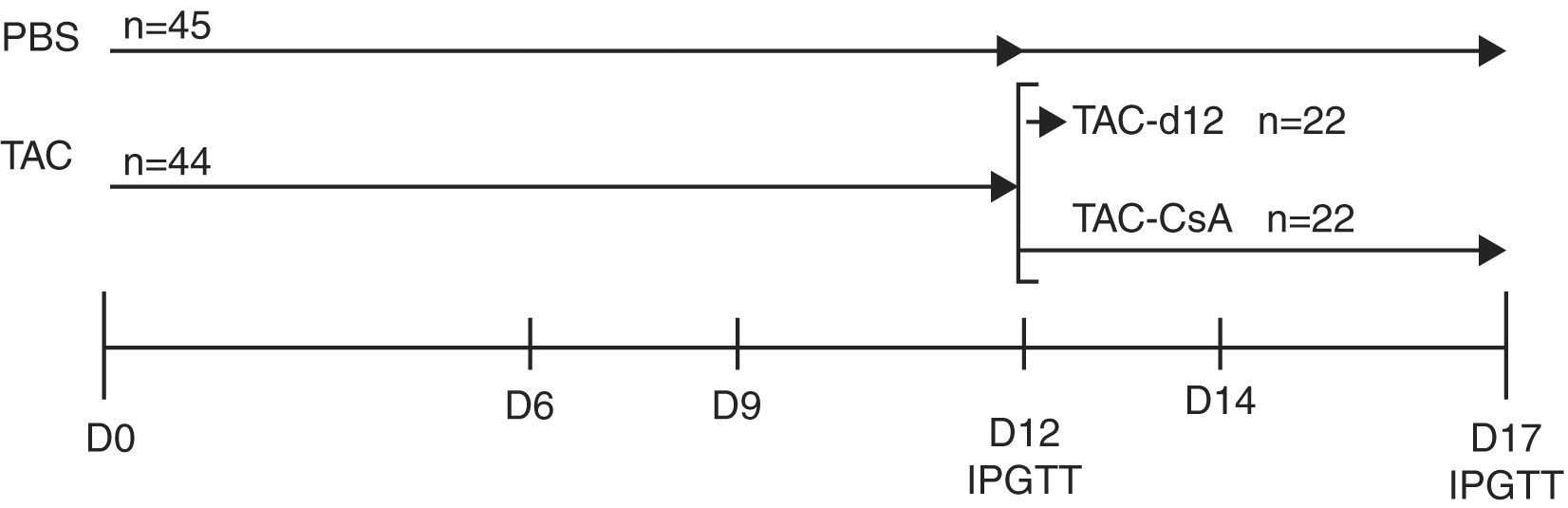
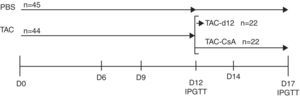
Experimental designBased on a prior study by our group,11 we induced diabetes with TAC in obese animals, and then we replaced TAC with CsA. To do this, we treated a group of OZR (n=44) with TAC for 11 days to induce diabetes, then, on day 12, the animals were segregated into two subgroups: (a) TAC-day-12 (Reference Group), formed by 22 animals which were subjected to an intraperitoneal glucose tolerance test (IPGTT), and were then sacrificed; and (b) TAC-CsA: formed by 22 animals, wherein TAC was replaced with CsA on day 12 of treatment, for 5 days; on day 17, the animals underwent IPGTT and were then sacrificed. The control group consisted of 45 animals that received the vehicle (PBS) for 12 days; IPGTT was performed on 23 of them, and they were sacrificed on day 12. Twenty-two of them received an IPGTT on day 17, and were then sacrificed (Fig. 1).
Time schedule for the experiment. The animals were treated with tacrolimus (0.3mg/kg/day) for 11 days, and they were randomly assigned to one of the following groups: (a) TAC-d12 (n=22), sacrificed on day 12, or (b) TAC-CSA (n=22), tacrolimus was replaced with CsA (2.5mg/kg/day) for 5 days. The un-treated control (PBS, n=23) received PBS for 11 days and was sacrificed on day 12, or received PBS for 17 days (PBS, n=22).
In our prior study,11 all the animals presented diabetes with severely high blood sugar after 12 days with TAC, which was reversed with the removal of the drug. Additionally, some animals died during the IPGTT on day 1211 due to severely high blood sugar. In this way, for this study, we considered that animals treated with TAC for 12 days already had established diabetes and, therefore, we did not extend the treatment with TAC to 17 days. To determine the length of treatment with CsA after the change (TAC-CsA), we carried out preliminary analyses in a subgroup of animals (n=5) with daily measurements of glucose levels while fasting after the change to CsA. All the animals showed normal glucose values (<126mg/dL) on the fifth day after the change to CsA, therefore, so we therefore decided to run the drug conversion study for five days after making the change to CsA.
The animals treated with TAC got a daily intraperitoneal injection of TAC (0.3mg/kg/day), and those who were changed to CsA received 2.5mg/kg/day. The dosages of TAC (0.3mg/kg/day) and CsA (2.5mg/kg/day) used were determined in prior experiments11 to obtain plasma levels similar to those used in clinical practice, both for CsA (200–250ng/mL) and for TAC (8–10ng/mL).
The animals were weighed daily, and their glucose levels while fasting were measured on days 12, 14 and 17. On day 12 of the group TAC-d12 (n=20) and on day 17 of the group TAC-CsA (n=15), underwent IPGTT with glucose (2g/kg), and glucose and insulin levels were measured at 0, 30, 60, and 120min. The animals with PBS also underwent IPGTT on days 12 and 17. All the animals were sacrificed with an intraperitoneal injection of pentobarbital sodium (50mg/kg).
On day 12, for groups PBS and TAC-d12, as well as on day 17 for PBS and TAC-CsA, the pancreas of 10 animals per group was removed for immunohistochemical and morphometrical analysis. The pancreas of another 10 animals of each group was removed for analysis of insulin gene expression.
Serum samples collected from the abdominal aorta were obtained to measure the levels of insulin and the biochemical parameters. The biochemical determinations were carried out in the central laboratory of the Hospital Universitario de Canarias.
DefinitionsDiabetes: blood sugar level while fasting ≥126mg/dL or >200mg/dL after 120min from the IPGTT (9). Pre-diabetes: abnormal blood sugar levels while fasting (ABSF): fasting glucose ≥100–125mg/dL or glucose intolerance (GIT): ≥140–199mg/dL 120min after IPGTT.9
Morphometrical and immunohistochemical analysisOn day 17, among 10 OZR per treatment group, the pancreas was removed and placed for 24h in 4% paraformaldehyde, and later included in paraffin. Serial 3μm-thick sections were made and placed on slides. For morphometrical analysis, the pancreas were stained with anti-insulin serum of guinea pig and revealed with immunologic methods based on biotin-streptavidin. The number of islets and the relative surface of the same based on the total surface of the pancreas were determined.
β-Cell proliferation was measured using the bromodeoxyuridine (BrdU) addition method (Roche, Switzerland). The animals were injected with BrdU (100mg/kg) 8h before being sacrificed. The pancreas was removed and processed as previously mentioned. The histologic sections were stained with antibodies for BrdU and insulin. Proliferation was measured as an estimate of the number of BrdU-positive cells based on the number of positive cells for insulin. The levels of apoptosis were measured with the TUNEL technique, and the same was carried out according to manufacturer specifications (Cell Death detection kit, POD) (Roche-Switzerland). The estimate of positive cells in TUNEL was adjusted to the positive surface for insulin. All the images were taken using an Olympus DP72 camera (Olympus, Tokyo-Japan) coupled to an Olympus DX41 microscope (Olympus, Tokyo, Japan), and later analysed using software ImageJ software (National Institute of Health).
RNA extractionThe islets of the remaining animals (n=10 per treatment) were isolated from the pancreas by means of digestion with collagenase.17 The islets used for RNA isolation were quickly frozen in D solution with thiocyanate guanidine. The total RNA was obtained with Chomczynski's method.18 We determined the purity and concentration of RNA with Nano-drop 2000 (Thermo-Fisher, Boston, MA).
Real-time PCRThe quantitation of relative abundance of mRNA of Ins2 was carried out by means of quantitative CRP (qCRP) using SYBR green as the detection method. The total RNA of the sample was retro-transcribed using a cDNA synthesis kit (Promega, Madison, WI) and the cDNA of Ins2 was amplified using specific primers. The sequence of oligonucleotides used was the following: 5′-TCATCCTCTGGGAGCCCCGC-3′ (sense primer); and 5′-GTTGCAGTAGTTCTCCAGTTGGT-3′ (anti-sense primer). The fluorescence increase during the CRP reaction was detected with the iQ5 system (Bio-Rad. Hercules, CA), and the gene expression was normalised with the reference genes beta-actin and SDHA. The data was analysed with qBASE software.19
Statistical analysisThe continuous variables are described as mean and standard deviation (SD) or mean and interquartile range when appropriate, while the dichotomous variables are represented with N and %. The groups were compared using a non-parameter Kruskal–Wallis test and the Mann–Whitney test, as necessary. For the qPCR data, we carried out a logarithmic transformation of the normalised gene expression, giving more symmetry to distribution, attributing equal weight to higher and lower gene expression conditions.20
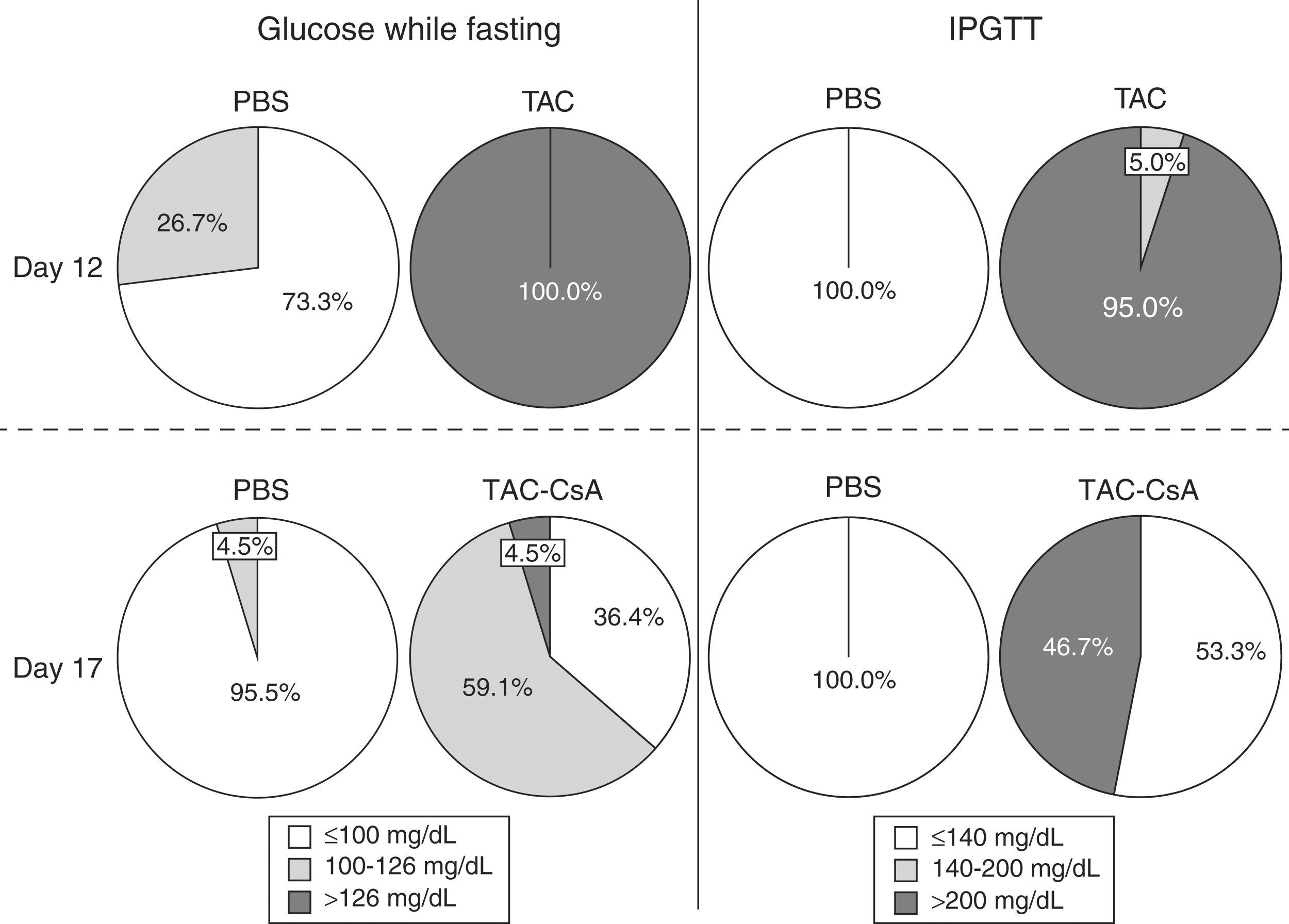
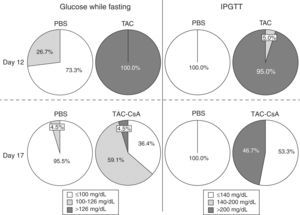
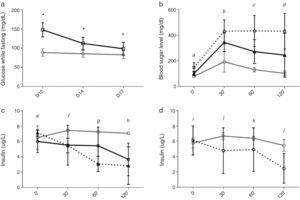
ResultsHomeostasis of glucose after 11 days with TacrolimusAccording to blood sugar levels while fasting, TAC induced diabetes in 100% of the animals by day 12 (Fig. 2). No animal with PBS developed diabetes, and only 26.7% (12/45) had pre-diabetes (Fig. 2). On day 12, 95% of the animals with TAC, which underwent IPGTT (n=20), presented glucose levels >200mg/dL after 120min (Fig. 2). Only one animal had blood sugar levels between 140 and 200mg/dL. No animal with PBS presented diabetes based on the IPGTT on day 12 (Fig. 2).
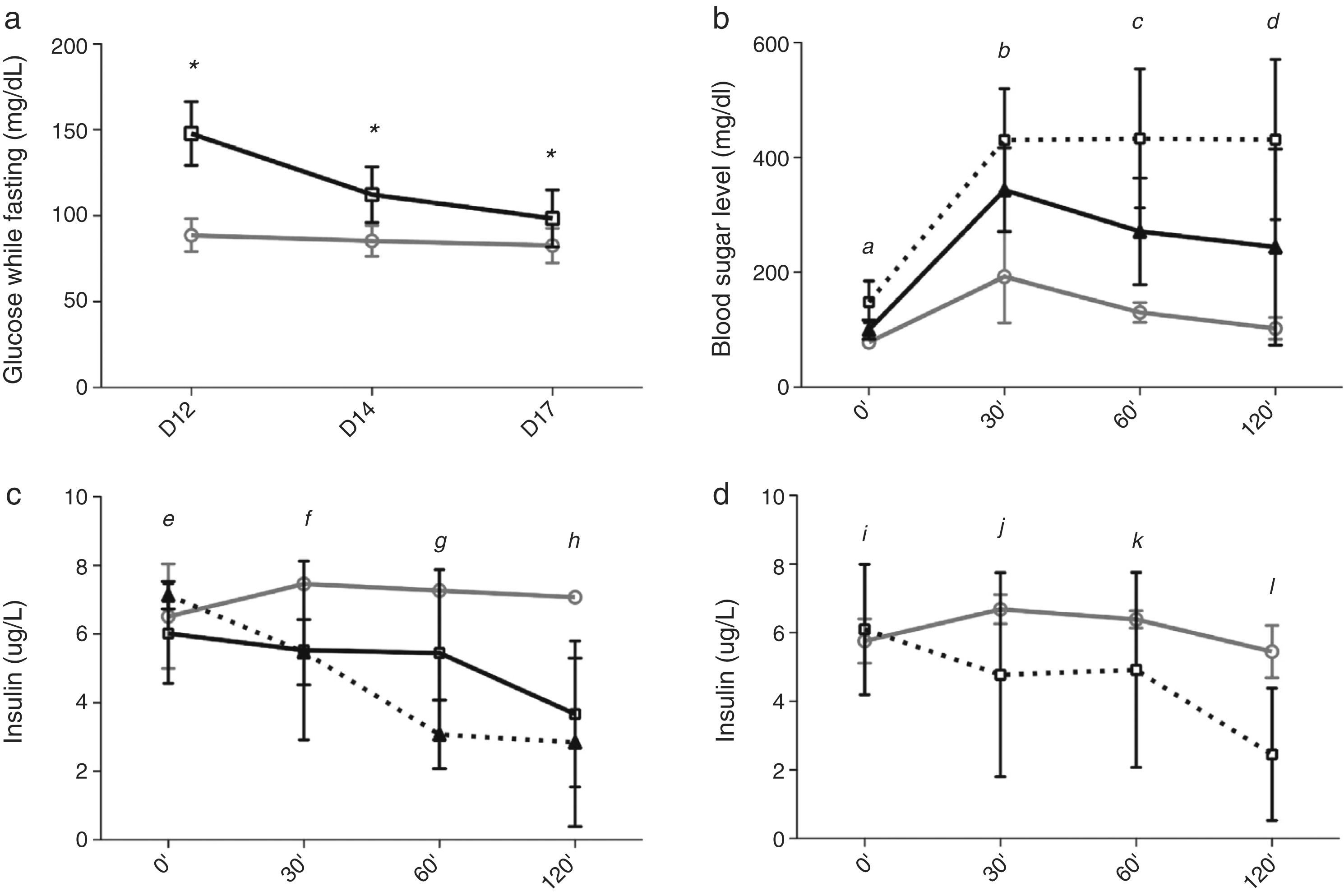
Homeostasis of glucose after the change from Tacrolimus to CyclosporineBetween days 12 and 17, after changing from TAC to CsA, fasting glucose levels decreased in the TAC-CsA group (Fig. 3a), and glucose levels on day 17 were significantly lower than compared to those of day 12 (p<0.001) (Fig. 3a). Based on fasting blood sugar levels, in the group TAC-CsA only one of 22 animals persisted with diabetes (4.5%), 13 of 22 (59.1%) had pre-diabetes, and 9 of 22 (36.4%) showed normal blood sugar while fasting (Fig. 2). Based on the IPGTT, 7 of 15 (46.7%) animals maintained diabetic values (2-h glucose >200mg/dL) in the group TAC-CsA, and 8 of 15 (53.3%) presented normal blood sugar (<140ml/dL) on day 17 (Fig. 2). At each point of the IPGTT, the animals of the TAC-CsA group presented higher blood sugar levels than those of the PBS group (Fig. 3b) but lower levels than those in the TAC-d12 group (Fig. 3b). Finally, on day 17, the animals with PBS presented normal IPGTT (Fig. 3b).
Progress of blood sugar levels and levels of insulin of OZR. (a) Blood sugar while fasting for PBS (grey line) and TAC (black line), (b) blood sugar levels, and (c) levels of insulin in the IPGTT on day 17. The grey line corresponds to PBS, and the black line to TAC-CsA. Black, discontinuous line TAC-d12, (d) levels of insulin in the IPGTT on day 17, for the animals of the group TAC-CsA with reversion of diabetes (grey line) or persistent diabetes (discontinuous line). (a) *: TAC-CsA vs PBS p≤0.0001. (b) a: all the comparisons p=0.0001; b: TAC-CsA or TAC-d12 vs PBS p=0.0001, TAC-CsA vs TAC-d12 p=0.005; c: TAC-CsA and TAC-d12 vs PBS p=0.0001; TAC-CsA vs TAC-d12 p=0.0001; d: TAC-CsA vs PBS p=0.012, TAC-d12 vs PBS p=0.0001, TAC-d12 vs TAC-CsA. p=0.002. (c) e: TAC-CsA or TAC-d12 vs PBS p>0.05, TAC-d12 vs TAC-CsA p=0.031, f: TAC-CsA vs PBS and vs TAC-CsA p>0.05, TAC-d12 vs PBS p=0.033; g: TAC-CsA vs PBS p=0.042, TAC-d12 vs PBS p=0.002, TAC-d12 vs TAC-CsA p=0.011; h: TAC-CsA and TAC-d12 vs PBS p≤0.0001, TAC-d12 vs TAC-CsA. p=0.002. (d) TAC-CsA-reversion of diabetes vs TAC-CsA-persistence of diabetes i, j and k: p>0.05, l: p=0.005.
During the IPGTT of day 17, the animals that had been changed from TAC to CsA did not show an increase in the early secretion of insulin (0–30min), but they did show lower insulin levels than the group with PBS (Fig. 3c), being comparable to the levels measured in the group TAC-d12 (Fig. 3c). However, during a sensitivity analysis, insulin secretion improved in the animal subgroup where diabetes was reversed after the change to CsA (n=8), as shown by a tendency towards higher levels of insulin after 30 and 60min, and significantly higher levels after 120min, compared with the animals where diabetes persisted in spite of the change to CsA (n=7) (Fig. 3d).
Finally, on day 12, TAC levels in the TAC-d12 were 8.37±3.94ng/mL; on day 17, the CsA levels in the TAC-CsA group were 508.18±67.85ng/mL, with residual levels of TAC of 2.46±0.90ng/mL.
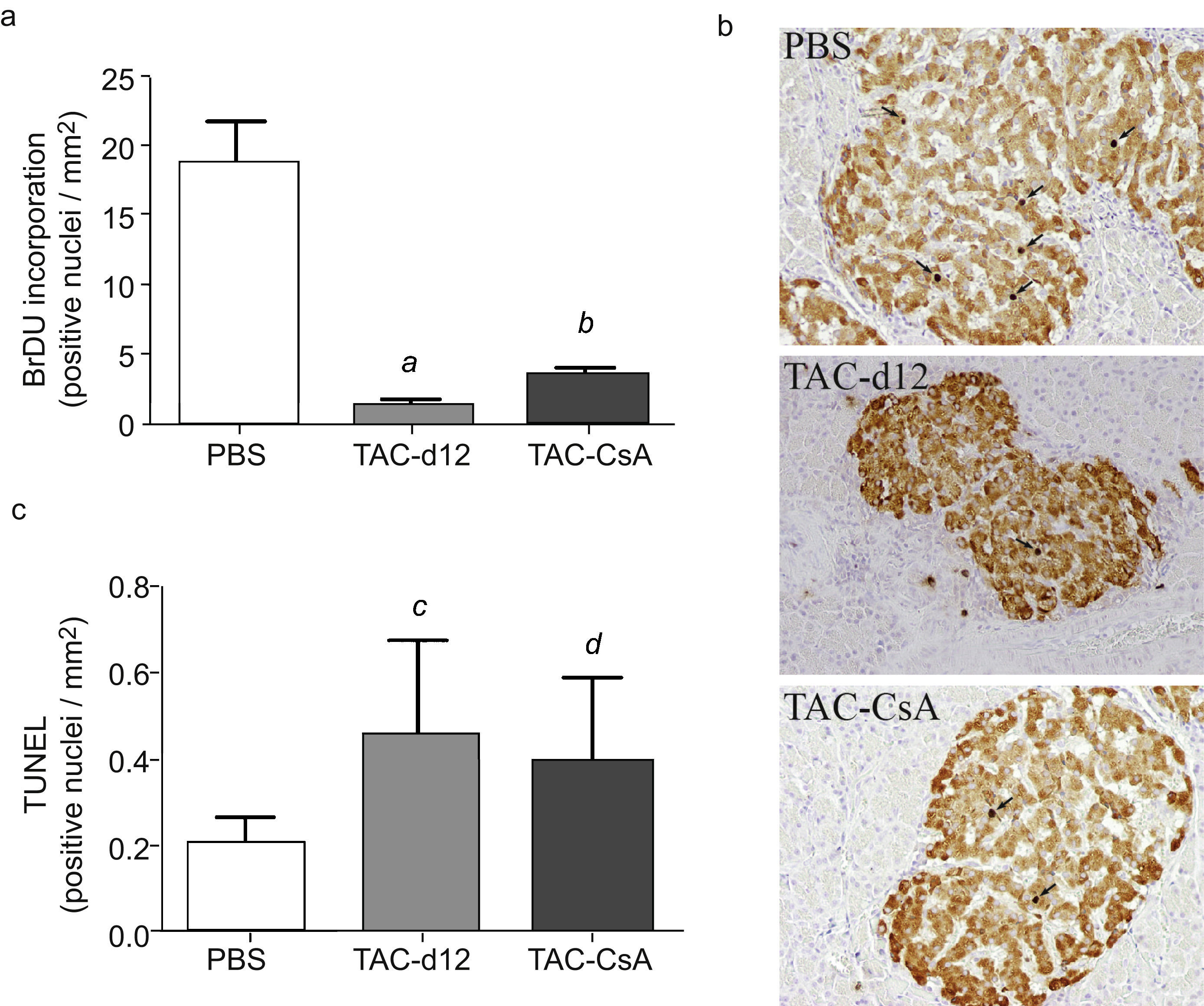
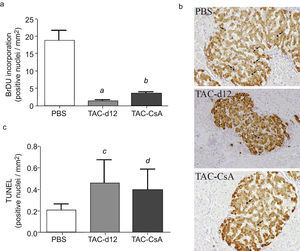
Proliferation of beta pancreatic cells, apoptosis and gene expression of insulin after 11 days with Tacrolimus and after the change to CyclosporineQuantitation of positive cells for insulin with bromodeoxyuridine showed a lower proliferation in TAC-d12 after 11 days of treatment than in the group with PBS (p=0.009) (Fig. 4a). When comparing the change from TAC to CsA (TAC-CsA) with the TAC-d12 group, the proliferation rate of β cells improved significantly (p=0.003) (Fig. 4a). No differences in apoptosis were observed between the two groups (Fig. 4b).
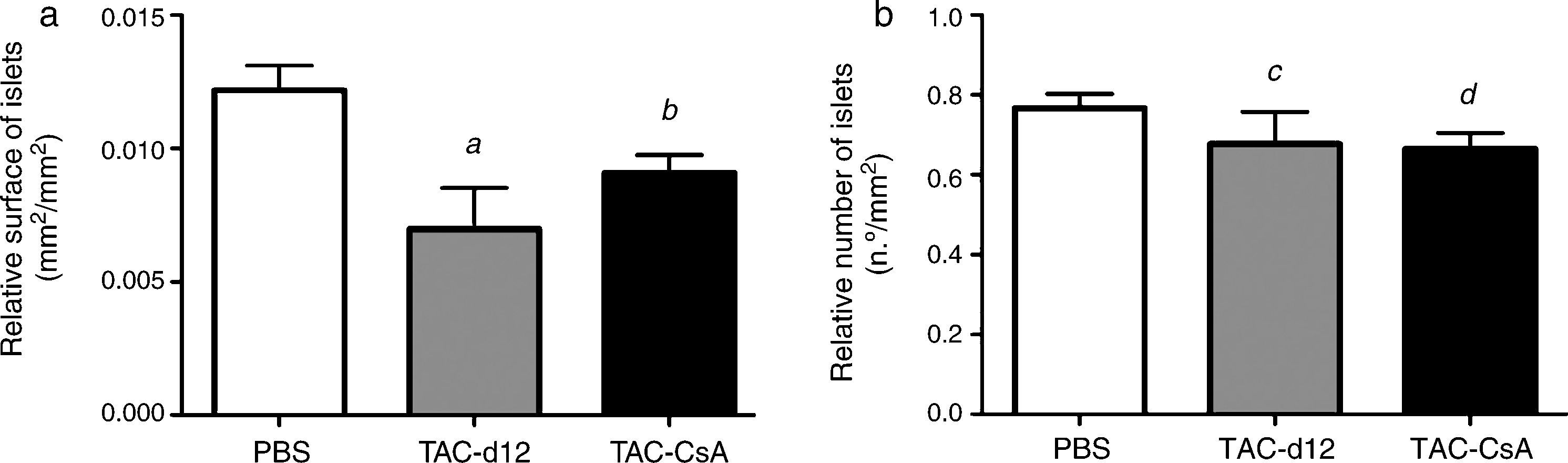
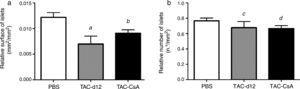
The relative islet surface was also lower in the TAC-d12 group when compared to the PBS group (p=0.011) (Fig. 5) but there were no differences with TAC-CsA. No differences were noted in the relative number of islets for either of the groups.
Morphometry of the OZR islets treated with PBS or TAC for 11 days (TAC-d12), or TAC for 11 days with change to CsA (TAC-CsA) for 5 days (n=10 per treatment). (a) Surface of islets adjusted to the total surface of pancreas. (b) Number of islets adjusted to mm2 of pancreas. The mean is represented±SD. (a) a: TAC-d12 vs PBS p=0.011. b: TAC-CsA vs PBS p=0.016 and vs TAC-d12 p=0.197. (b) c: TAC-d12 vs PBS p=0.523. d: TAC-CsA vs PBS p=0.078 and vs TAC-d12 p=0.892.
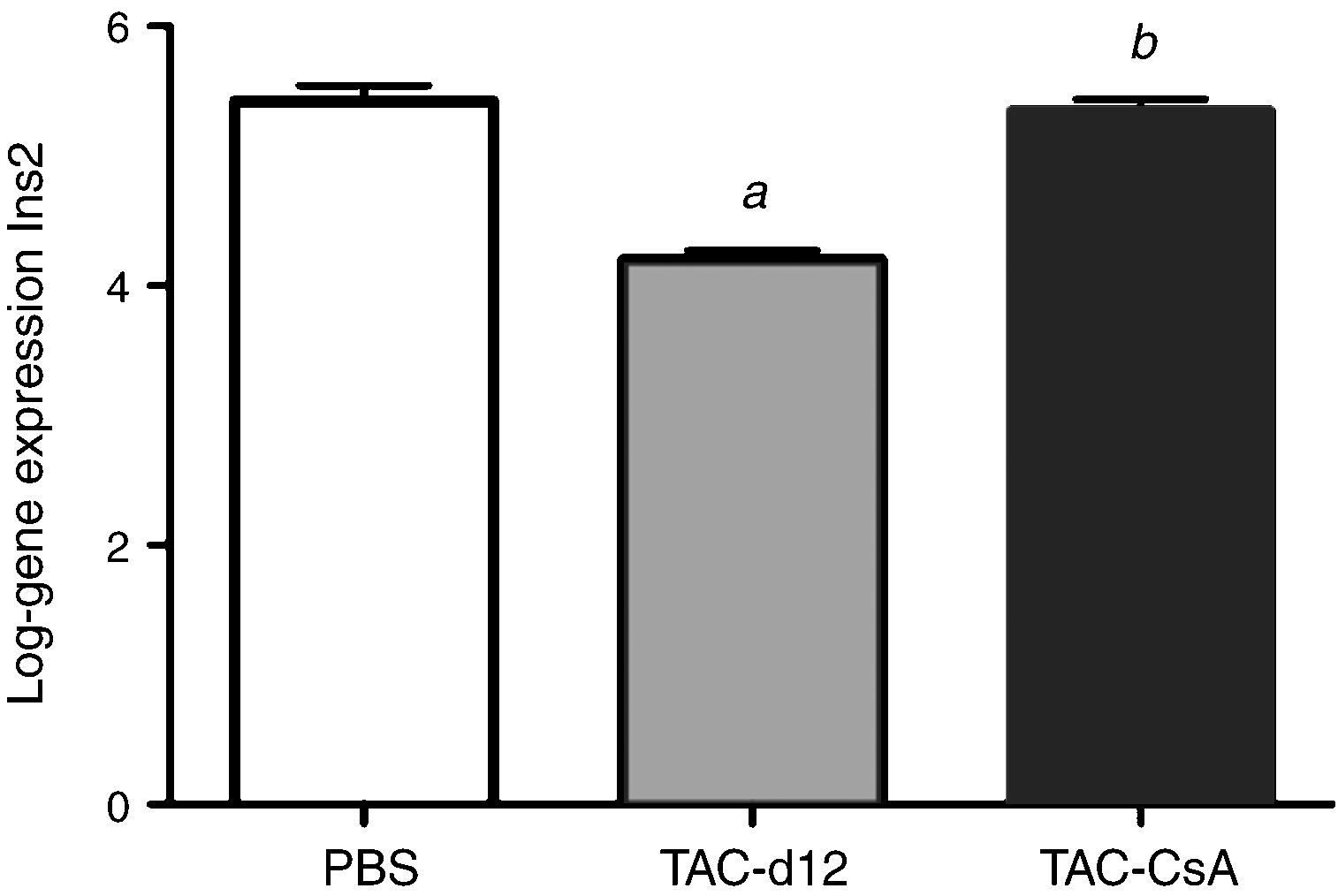
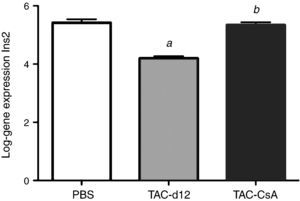
Eleven days with Tacrolimus (TAC-d12) significantly reduced the expression of the Ins2 gene compared to the group with PBS (p≤0.0001) (Fig. 6). Five days after the change to CsA (TAC-CsA), Ins2 gene expression returned to values comparable to those of PBS (p=0.755) and it was significantly increased versus TAC-d12 (p≤0.0001) (Fig. 6).
DiscussionIn this model of diabetes induced by tacrolimus in animals with resistance to insulin, we have observed that the change to Cyclosporine-A leads to: (1) improved blood sugar levels, both fasting and during IPGTT; (2) a reversion of diabetes in 53.3% of the animals; (3) an improvement in β-cell proliferation; and, finally, (4) an increase in insulin gene expression.
The obese Zucker rat (OZR) is a known model of insulin resistance. We have previously observed that in OZR, the greatest diabetogenic effect of TAC compared to CsA is determined by insulin resistance.11 For this study, the objective of which was to better understand the reversion mechanisms of NODAT, we used OZR instead of insulin-sensitive model animals such as Sprague Dawley, Wistar, or TZR. We have confirmed our prior findings, wherein the administration of TAC during 11 days induced diabetes in 100% of the insulin-resistant animals, diagnosed either by blood sugar levels while fasting or by IPGTT (Figs. 2 and 3).
Our main finding is that in diabetes induced by TAC, the change to CsA leads to an improvement in the metabolism of glucose and to a reversibility of diabetes in 50% of the animals (Fig. 2). This reversibility was noted only 5 days after the change to CsA (Fig. 3a and b). However, it was not complete, since 59.1% of the animals showed blood sugar levels within the pre-diabetes range (due to baseline blood sugar while fasting), and in 46.7% the diabetes persisted according to the IPGTT results (blood sugar after 120min >200mg/dL) (Fig. 2). In this way, the changes to homeostasis of glucose, although relevant if compared to the diabetes established in the group TAC, did not represent a total reversibility of the diabetes induced by the drug.
The causes for glucose metabolism improvement after substituting TAC for CsA are not clear, but they could be in part related to an increase in the proliferation of β cell (Fig. 4). We have previously observed that TAC is a stronger inhibitor of β cell proliferation than CsA.11 With this, it is plausible that the substitution of a strong proliferation inhibitor (TAC) for a less stronger one (CsA) will help the β cell to recover, to a certain extent, its proliferative capability, and, as a consequence, to better manage the high blood sugar levels. Currently, β cells are considered more resistant to damage than was previously believed, with high recovery capability in the face of a toxic environment.21 In our study, a reduction in the mean surface of pancreatic islets after 11 days of treatment with TAC is particularly remarkable, which does not correspond to an equivalent increase in the apoptosis of beta cell. In this regard, numerous efforts have been made to determine the mechanisms causing a decrease in the function and in the number of beta cells among patients with DMT2. New evidence suggests that these changes could be due to de-differentiation or trans-differentiation processes in the cells, and not to apoptosis processes, which suggests a new concept in the pathogenesis of beta cell dysfunction.22 These new hypotheses would be in agreement with our model, wherein a loss of the identity marker of a beta cell such as insulin is observed, but, on the contrary, no apoptosis increase is noted. Additionally, the quick recovery observed after the drug change, as well as the previously observed quick withdrawal,11 supports the notion of a loss of function and identity of the beta cell rather than a process of cellular death. In any event, future experiments are required to confirm these hypotheses for this model of diabetes induced by inhibitors of calcineurin.
We have also noted in prior studies that TAC is a stronger inhibitor of the gene Ins2 expression than CsA.11 According to these data, the expression of gene Ins2 (homologous to the human proinsulin gene) after being exposed to TAC (TAC-d12) for 11 days was significantly lower than in animals who only received the vehicle (PBS) for 17 days (Fig. 6). The increase in the expression of gene Ins2 after the change to CsA was significantly higher when compared to the group that was only administered TAC (p≤0.0001 vs TAC-d12), which suggests that an improvement in the expression of the gene Ins2 could be related to better management of glucose after the change to CsA.
The molecular mechanisms involved in the increase of proliferation of β cell associated with the change of TAC to CsA are, to the best of our knowledge, unknown. The fact that TAC is a stronger inhibitor of calcineurin than CsA23 could explain the greater impact of the first on the decrease of calcineurin-dependent transcription factors such as the nuclear factor of T cells (NFAT), the element of response to cAMP (CREB), and the CREB transducer (TORC2), which are crucial for the proliferation of β cell.24–26
Our results are consistent with the few retrospective studies carried out on humans.10 Ghisdal et al. observed 42% of reversibility of PTDM with the change of TAC to CsA in 34 patients, and 0% in 20 patients who continued with TAC.10 In said work, the authors also quoted another clinical study with similar findings. However, these works present a post hoc design, and low statistical strength to prove efficacy and security. This work on animals with insulin resistance provides information on the mechanisms and the way in which the improvement of glucose metabolism is associated to an early change to CsA, once the diabetes induced by TAC is established. Future clinical trials will be required to establish the role that this strategy could have at the time of minimising damage caused to the β cell while establishing the PTDM.
Our study has limitations. The first is the application of data obtained in an animal model to clinical research. However, the coincidences between this work and retrospective works published on humans10 could hint at the usefulness of this animal model for the study of the impact of changing from TAC to CsA on the metabolism of glucose and on the biology of the β cell. The second limitation is with regards to the application of human criteria such as pre-diabetes and diabetes to an animal model. However, this is a standard in basic research,26–29 and, to the best of our knowledge, there is no clear consensus for these definitions on rodents. Another limitation is related to the length of the change; 5 days of change to CsA could be too few, but during those days, the glucose levels while fasting showed significant improvements by day 17. Further, our results show that it is sufficient time to dilute the toxic effect of TAC, and, therefore, to rule out the possibility of an accumulative effect of both drugs on the β cell. The effect which could occur on the homeostasis of glucose following a longer exposure to CsA should be established in the future. In this study, we used relatively high levels of CsA, which could have increased the risk of diabetes in animals that received this drug. However, the use of lower levels could only have increased the differences with the group of tacrolimus. Lastly, in this animal model, the diabetes was established after a short period of exposure to the drug, and the experiment was not designed to assess the reversibility of diabetes after a long period of time, which should be researched.
In summary, we can conclude that in diabetes induced by Tacrolimus, the change to Cyclosporine improves the homeostasis of glucose, which is related to an increase in the proliferation of β cell and possibly to an increase in the expression of the insulin gene. The ad-hoc assessment of this effect on patients with PTDM should be studied.
FundingThe authors would like to thank the project IMBRAIN (FP7-RE6-POT-2012-CT2012-31637-IMBRAIN), founded under the 7° Frameworks Programme. They thank the Instituto de Salud Carlos III (Health Investment Fund: HIF) for the following financing: PI 07/0732, REDINREN RD/0021/0008 and PI10/02428. They also give their acknowledgements to the funds of IRSIN (Instituto Reina Sofía de Investigación) and to Funds FEDER.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rodríguez-Rodríguez AE, Triñanes J, Porrini E, Velázquez-García S, Fumero C, Vega-Prieto MJ, et al. Cambios en la homeostasis de la glucosa y la proliferación de la célula beta pancreática tras el cambio a ciclosporina en la diabetes inducida por tacrolimus. Nefrologia. 2015;35:264–272.